198 Topics in Current Chemistry Editorial Board: A. de Meijere KN ...
198 Topics in Current Chemistry Editorial Board: A. de Meijere KN ...
198 Topics in Current Chemistry Editorial Board: A. de Meijere KN ...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
42 J.P. Glusker<br />
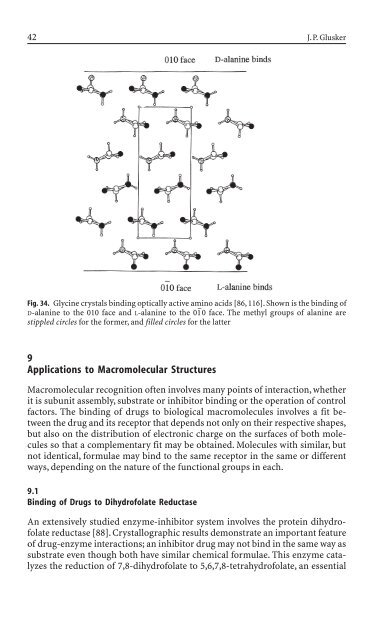
Fig. 34. Glyc<strong>in</strong>e crystals b<strong>in</strong>d<strong>in</strong>g optically active am<strong>in</strong>o acids [86, 116]. Shown is the b<strong>in</strong>d<strong>in</strong>g of<br />
D-alan<strong>in</strong>e to the 010 face and L-alan<strong>in</strong>e to the 01 – 0 face. The methyl groups of alan<strong>in</strong>e are<br />
stippled circles for the former, and filled circles for the latter<br />
9<br />
Applications to Macromolecular Structures<br />
Macromolecular recognition often <strong>in</strong>volves many po<strong>in</strong>ts of <strong>in</strong>teraction, whether<br />
it is subunit assembly, substrate or <strong>in</strong>hibitor b<strong>in</strong>d<strong>in</strong>g or the operation of control<br />
factors. The b<strong>in</strong>d<strong>in</strong>g of drugs to biological macromolecules <strong>in</strong>volves a fit between<br />
the drug and its receptor that <strong>de</strong>pends not only on their respective shapes,<br />
but also on the distribution of electronic charge on the surfaces of both molecules<br />
so that a complementary fit may be obta<strong>in</strong>ed. Molecules with similar, but<br />
not i<strong>de</strong>ntical, formulae may b<strong>in</strong>d to the same receptor <strong>in</strong> the same or different<br />
ways, <strong>de</strong>pend<strong>in</strong>g on the nature of the functional groups <strong>in</strong> each.<br />
9.1<br />
B<strong>in</strong>d<strong>in</strong>g of Drugs to Dihydrofolate Reductase<br />
An extensively studied enzyme-<strong>in</strong>hibitor system <strong>in</strong>volves the prote<strong>in</strong> dihydrofolate<br />
reductase [88]. Crystallographic results <strong>de</strong>monstrate an important feature<br />
of drug-enzyme <strong>in</strong>teractions; an <strong>in</strong>hibitor drug may not b<strong>in</strong>d <strong>in</strong> the same way as<br />
substrate even though both have similar chemical formulae. This enzyme catalyzes<br />
the reduction of 7,8-dihydrofolate to 5,6,7,8-tetrahydrofolate, an essential