198 Topics in Current Chemistry Editorial Board: A. de Meijere KN ...
198 Topics in Current Chemistry Editorial Board: A. de Meijere KN ...
198 Topics in Current Chemistry Editorial Board: A. de Meijere KN ...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
176 M.R. Caira<br />
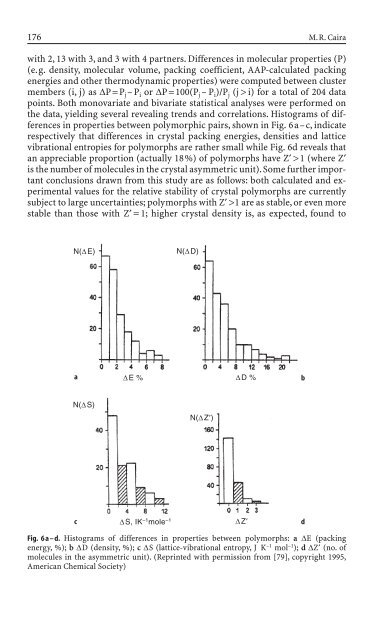
with 2, 13 with 3, and 3 with 4 partners. Differences <strong>in</strong> molecular properties (P)<br />
(e.g. <strong>de</strong>nsity, molecular volume, pack<strong>in</strong>g coefficient, AAP-calculated pack<strong>in</strong>g<br />
energies and other thermodynamic properties) were computed between cluster<br />
members (i, j) as DP=P j–P i or DP=100(P j –P i)/P j (j > i) for a total of 204 data<br />
po<strong>in</strong>ts. Both monovariate and bivariate statistical analyses were performed on<br />
the data, yield<strong>in</strong>g several reveal<strong>in</strong>g trends and correlations. Histograms of differences<br />
<strong>in</strong> properties between polymorphic pairs,shown <strong>in</strong> Fig. 6a–c,<strong>in</strong>dicate<br />
respectively that differences <strong>in</strong> crystal pack<strong>in</strong>g energies, <strong>de</strong>nsities and lattice<br />
vibrational entropies for polymorphs are rather small while Fig. 6d reveals that<br />
an appreciable proportion (actually 18%) of polymorphs have Z¢ >1 (where Z¢<br />
is the number of molecules <strong>in</strong> the crystal asymmetric unit).Some further important<br />
conclusions drawn from this study are as follows: both calculated and experimental<br />
values for the relative stability of crystal polymorphs are currently<br />
subject to large uncerta<strong>in</strong>ties; polymorphs with Z¢ >1 are as stable, or even more<br />
stable than those with Z¢ = 1; higher crystal <strong>de</strong>nsity is, as expected, found to<br />
N(DE)<br />
a DE %<br />
DD %<br />
b<br />
N(DS)<br />
N(DD)<br />
N(DZ¢)<br />
c DS, IK d<br />
–1mole –1 DZ¢<br />
Fig. 6 a – d. Histograms of differences <strong>in</strong> properties between polymorphs: a DE (pack<strong>in</strong>g<br />
energy, %); b DD (<strong>de</strong>nsity, %); c DS (lattice-vibrational entropy, J K –1 mol –1 ); d DZ¢ (no. of<br />
molecules <strong>in</strong> the asymmetric unit). (Repr<strong>in</strong>ted with permission from [79], copyright 1995,<br />
American Chemical Society)