- Page 1 and 2: 198 Topics in Current Chemistry Edi
- Page 3 and 4: Design of Organic Solids Volume Edi
- Page 5 and 6: Volume Editors Prof. Dr. Edwin Webe
- Page 7 and 8: VIII preface tion at an amazing lev
- Page 9 and 10: Contents of Volume 196 Carbon Rich
- Page 11 and 12: 2 J.P. Glusker 5 Interactions of Pl
- Page 13 and 14: 4 J.P. Glusker Perturbations to the
- Page 15 and 16: 6 J.P. Glusker 2.1 Sources of Cryst
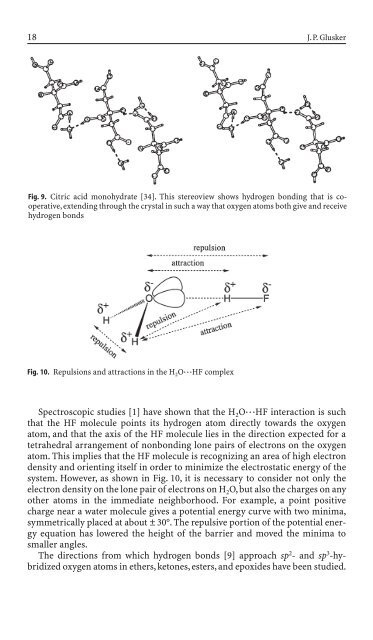
- Page 17 and 18: 8 J.P. Glusker other induced, will
- Page 19 and 20: 10 J.P. Glusker The forces here hav
- Page 21 and 22: 12 J.P. Glusker lographic structure
- Page 23 and 24: 14 J.P. Glusker a c Fig. 6 a - c. D
- Page 25: 16 J.P. Glusker hydrogen bond H◊
- Page 29 and 30: 20 J.P. Glusker peptide plane and a
- Page 31 and 32: 22 J.P. Glusker 4.5 F◊◊◊H Int
- Page 33 and 34: 24 J.P. Glusker rine-containing com
- Page 35 and 36: 26 J.P. Glusker Fig. 17. The packin
- Page 37 and 38: 28 J.P. Glusker bind to a metal ion
- Page 39 and 40: 30 J.P. Glusker As for carboxylate
- Page 41 and 42: 32 J.P. Glusker Fig. 24. The magnes
- Page 43 and 44: 34 J.P. Glusker structures of magne
- Page 45 and 46: 36 J.P. Glusker b a Fig. 28 a, b. A
- Page 47 and 48: 38 J.P. Glusker Fig. 30. Complexati
- Page 49 and 50: 40 J.P. Glusker 7.1 Descriptions of
- Page 51 and 52: 42 J.P. Glusker Fig. 34. Glycine cr
- Page 53 and 54: 44 J.P. Glusker bonding capabilitie
- Page 55 and 56: 46 J.P. Glusker 2. Non-intercalativ
- Page 57 and 58: 48 J.P. Glusker d Fig. 37 d. The ch
- Page 59 and 60: 50 J.P. Glusker Fig. 39. Hydrogen-b
- Page 61 and 62: 52 J.P. Glusker 72, 104], but other
- Page 63 and 64: 54 J.P. Glusker 23. Cody V, Murray-
- Page 65 and 66: 56 J.P. Glusker: Directional Aspect
- Page 67 and 68: 58 A. Nangia · G.R. Desiraju 7 Sup
- Page 69 and 70: 60 A. Nangia · G.R. Desiraju elect
- Page 71 and 72: 62 A. Nangia · G.R. Desiraju 14 15
- Page 73 and 74: 64 A. Nangia · G.R. Desiraju [23]
- Page 75 and 76: 66 A. Nangia · G.R. Desiraju struc
- Page 77 and 78:
68 A. Nangia · G.R. Desiraju Fig.
- Page 79 and 80:
70 A. Nangia · G.R. Desiraju -NH 2
- Page 81 and 82:
72 A. Nangia · G.R. Desiraju ingfu
- Page 83 and 84:
74 A. Nangia · G.R. Desiraju and r
- Page 85 and 86:
76 A. Nangia · G.R. Desiraju bonds
- Page 87 and 88:
78 A. Nangia · G.R. Desiraju 2.86-
- Page 89 and 90:
80 A. Nangia · G.R. Desiraju from
- Page 91 and 92:
82 A. Nangia · G.R. Desiraju ted a
- Page 93 and 94:
84 A. Nangia · G.R. Desiraju envir
- Page 95 and 96:
86 A. Nangia · G.R. Desiraju PYRAZ
- Page 97 and 98:
88 A. Nangia · G.R. Desiraju napht
- Page 99 and 100:
90 A. Nangia · G.R. Desiraju b c F
- Page 101 and 102:
92 A. Nangia · G.R. Desiraju all a
- Page 103 and 104:
94 A. Nangia · G.R. Desiraju 42. J
- Page 105 and 106:
Hydrogen-Bonded Ribbons, Tapes and
- Page 107 and 108:
Hydrogen-Bonded Ribbons, Tapes and
- Page 109 and 110:
Hydrogen-Bonded Ribbons, Tapes and
- Page 111 and 112:
Hydrogen-Bonded Ribbons, Tapes and
- Page 113 and 114:
Hydrogen-Bonded Ribbons, Tapes and
- Page 115 and 116:
Hydrogen-Bonded Ribbons, Tapes and
- Page 117 and 118:
Hydrogen-Bonded Ribbons, Tapes and
- Page 119 and 120:
Hydrogen-Bonded Ribbons, Tapes and
- Page 121 and 122:
Hydrogen-Bonded Ribbons, Tapes and
- Page 123 and 124:
Hydrogen-Bonded Ribbons, Tapes and
- Page 125 and 126:
Hydrogen-Bonded Ribbons, Tapes and
- Page 127 and 128:
Hydrogen-Bonded Ribbons, Tapes and
- Page 129 and 130:
Hydrogen-Bonded Ribbons, Tapes and
- Page 131 and 132:
Hydrogen-Bonded Ribbons, Tapes and
- Page 133 and 134:
Hydrogen-Bonded Ribbons, Tapes and
- Page 135 and 136:
Hydrogen-Bonded Ribbons, Tapes and
- Page 137 and 138:
Hydrogen-Bonded Ribbons, Tapes and
- Page 139 and 140:
132 Y. Aoyama 4 Catalysis . . . . .
- Page 141 and 142:
134 Y. Aoyama 2.2 Symmetry-Controll
- Page 143 and 144:
136 Y. Aoyama A trigonal centre is
- Page 145 and 146:
138 Y. Aoyama 18 19 Fig. 5. 2D net
- Page 147 and 148:
140 Y. Aoyama 24 ded (O-H◊◊◊O
- Page 149 and 150:
142 Y. Aoyama 2.4 Interaction-Suppo
- Page 151 and 152:
144 Y. Aoyama In the presence of a
- Page 153 and 154:
146 Y. Aoyama clusion compounds (se
- Page 155 and 156:
148 Y. Aoyama are intriguing guests
- Page 157 and 158:
150 Y. Aoyama The tuning or enginee
- Page 159 and 160:
152 Y. Aoyama Fig. 16. Binding isot
- Page 161 and 162:
154 Y. Aoyama The desorption of the
- Page 163 and 164:
156 Y. Aoyama Fig. 17. Suggested me
- Page 165 and 166:
158 Y. Aoyama 5 Concluding Remarks
- Page 167 and 168:
160 Y. Aoyama 4. Hölderich W, Hess
- Page 169 and 170:
Crystalline Polymorphism of Organic
- Page 171 and 172:
Crystalline Polymorphism of Organic
- Page 173 and 174:
Crystalline Polymorphism of Organic
- Page 175 and 176:
Crystalline Polymorphism of Organic
- Page 177 and 178:
Crystalline Polymorphism of Organic
- Page 179 and 180:
Crystalline Polymorphism of Organic
- Page 181 and 182:
Crystalline Polymorphism of Organic
- Page 183 and 184:
Crystalline Polymorphism of Organic
- Page 185 and 186:
Crystalline Polymorphism of Organic
- Page 187 and 188:
Crystalline Polymorphism of Organic
- Page 189 and 190:
Crystalline Polymorphism of Organic
- Page 191 and 192:
Crystalline Polymorphism of Organic
- Page 193 and 194:
Crystalline Polymorphism of Organic
- Page 195 and 196:
Crystalline Polymorphism of Organic
- Page 197 and 198:
Crystalline Polymorphism of Organic
- Page 199 and 200:
Crystalline Polymorphism of Organic
- Page 201 and 202:
Crystalline Polymorphism of Organic
- Page 203 and 204:
Crystalline Polymorphism of Organic
- Page 205 and 206:
Crystalline Polymorphism of Organic
- Page 207 and 208:
Crystalline Polymorphism of Organic
- Page 209 and 210:
Crystalline Polymorphism of Organic
- Page 211 and 212:
Crystalline Polymorphism of Organic
- Page 213 and 214:
Crystalline Polymorphism of Organic
- Page 215 and 216:
Author Index Volumes 151-198 Author
- Page 217 and 218:
Author Index Volumes 151 - 198 2 1
- Page 219 and 220:
Author Index Volumes 151 - 198 213
- Page 221 and 222:
Author Index Volumes 151 - 198 215
- Page 223 and 224:
Author Index Volumes 151 - 198 2 ]
- Page 225 and 226:
Author Index Volumes 151 - 198 219
- Page 227 and 228:
Springer and the environment At Spr