198 Topics in Current Chemistry Editorial Board: A. de Meijere KN ...
198 Topics in Current Chemistry Editorial Board: A. de Meijere KN ...
198 Topics in Current Chemistry Editorial Board: A. de Meijere KN ...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
168 M.R. Caira<br />
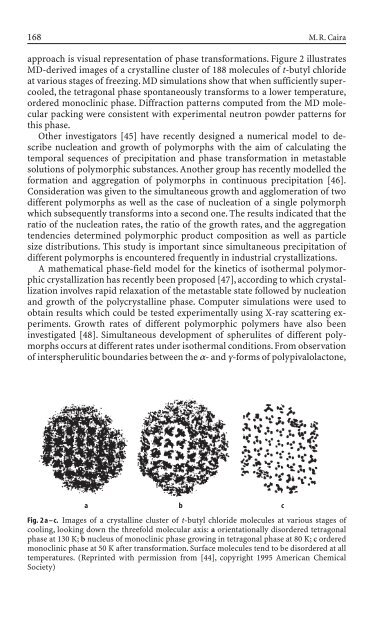
approach is visual representation of phase transformations. Figure 2 illustrates<br />
MD-<strong>de</strong>rived images of a crystall<strong>in</strong>e cluster of 188 molecules of t-butyl chlori<strong>de</strong><br />
at various stages of freez<strong>in</strong>g. MD simulations show that when sufficiently supercooled,<br />
the tetragonal phase spontaneously transforms to a lower temperature,<br />
or<strong>de</strong>red monocl<strong>in</strong>ic phase. Diffraction patterns computed from the MD molecular<br />
pack<strong>in</strong>g were consistent with experimental neutron pow<strong>de</strong>r patterns for<br />
this phase.<br />
Other <strong>in</strong>vestigators [45] have recently <strong>de</strong>signed a numerical mo<strong>de</strong>l to <strong>de</strong>scribe<br />
nucleation and growth of polymorphs with the aim of calculat<strong>in</strong>g the<br />
temporal sequences of precipitation and phase transformation <strong>in</strong> metastable<br />
solutions of polymorphic substances. Another group has recently mo<strong>de</strong>lled the<br />
formation and aggregation of polymorphs <strong>in</strong> cont<strong>in</strong>uous precipitation [46].<br />
Consi<strong>de</strong>ration was given to the simultaneous growth and agglomeration of two<br />
different polymorphs as well as the case of nucleation of a s<strong>in</strong>gle polymorph<br />
which subsequently transforms <strong>in</strong>to a second one. The results <strong>in</strong>dicated that the<br />
ratio of the nucleation rates, the ratio of the growth rates, and the aggregation<br />
ten<strong>de</strong>ncies <strong>de</strong>term<strong>in</strong>ed polymorphic product composition as well as particle<br />
size distributions. This study is important s<strong>in</strong>ce simultaneous precipitation of<br />
different polymorphs is encountered frequently <strong>in</strong> <strong>in</strong>dustrial crystallizations.<br />
A mathematical phase-field mo<strong>de</strong>l for the k<strong>in</strong>etics of isothermal polymorphic<br />
crystallization has recently been proposed [47], accord<strong>in</strong>g to which crystallization<br />
<strong>in</strong>volves rapid relaxation of the metastable state followed by nucleation<br />
and growth of the polycrystall<strong>in</strong>e phase. Computer simulations were used to<br />
obta<strong>in</strong> results which could be tested experimentally us<strong>in</strong>g X-ray scatter<strong>in</strong>g experiments.<br />
Growth rates of different polymorphic polymers have also been<br />
<strong>in</strong>vestigated [48]. Simultaneous <strong>de</strong>velopment of spherulites of different polymorphs<br />
occurs at different rates un<strong>de</strong>r isothermal conditions. From observation<br />
of <strong>in</strong>terspherulitic boundaries between the a- and g-forms of polypivalolactone,<br />
a b c<br />
Fig. 2 a – c. Images of a crystall<strong>in</strong>e cluster of t-butyl chlori<strong>de</strong> molecules at various stages of<br />
cool<strong>in</strong>g, look<strong>in</strong>g down the threefold molecular axis: a orientationally disor<strong>de</strong>red tetragonal<br />
phase at 130 K; b nucleus of monocl<strong>in</strong>ic phase grow<strong>in</strong>g <strong>in</strong> tetragonal phase at 80 K; c or<strong>de</strong>red<br />
monocl<strong>in</strong>ic phase at 50 K after transformation. Surface molecules tend to be disor<strong>de</strong>red at all<br />
temperatures. (Repr<strong>in</strong>ted with permission from [44], copyright 1995 American Chemical<br />
Society)