198 Topics in Current Chemistry Editorial Board: A. de Meijere KN ...
198 Topics in Current Chemistry Editorial Board: A. de Meijere KN ...
198 Topics in Current Chemistry Editorial Board: A. de Meijere KN ...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
Crystall<strong>in</strong>e Polymorphism of Organic Compounds 173<br />
(~ 3.2 Å) of the respective layers shown <strong>in</strong> Fig. 4. This example conveys an i<strong>de</strong>a<br />
of the variations <strong>in</strong> polymorphic structural arrangements that are possible with<br />
molecules conta<strong>in</strong><strong>in</strong>g hydrogen bond<strong>in</strong>g functionalities.<br />
Dur<strong>in</strong>g the last few years, the <strong>de</strong>velopment of graph set analysis [16] has<br />
greatly facilitated the visualisation and comparison of polymorphic structures.<br />
Here, hydrogen bond<strong>in</strong>g networks are classified as belong<strong>in</strong>g to one of four<br />
dist<strong>in</strong>ct patterns, each specified by a <strong>de</strong>signator (G <strong>in</strong> general): <strong>in</strong>tramolecular<br />
(S), cha<strong>in</strong>s (C), r<strong>in</strong>gs (R), or other f<strong>in</strong>ite patterns (D). Further specification of the<br />
number of donor (d) and acceptor (a) atoms as well as the total number of atoms<br />
(n) compris<strong>in</strong>g the pattern yields a concise and <strong>in</strong>formative <strong>de</strong>scription of the<br />
hydrogen bond<strong>in</strong>g arrangement, G a d(n).A simple illustration of the use of graph<br />
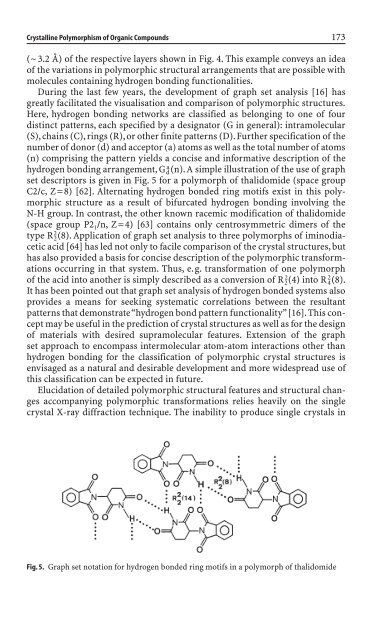
set <strong>de</strong>scriptors is given <strong>in</strong> Fig. 5 for a polymorph of thalidomi<strong>de</strong> (space group<br />
C2/c, Z=8) [62]. Alternat<strong>in</strong>g hydrogen bon<strong>de</strong>d r<strong>in</strong>g motifs exist <strong>in</strong> this polymorphic<br />
structure as a result of bifurcated hydrogen bond<strong>in</strong>g <strong>in</strong>volv<strong>in</strong>g the<br />
N-H group. In contrast, the other known racemic modification of thalidomi<strong>de</strong><br />
(space group P2 1/n, Z=4) [63] conta<strong>in</strong>s only centrosymmetric dimers of the<br />
type R 2 2(8). Application of graph set analysis to three polymorphs of im<strong>in</strong>odiacetic<br />
acid [64] has led not only to facile comparison of the crystal structures, but<br />
has also provi<strong>de</strong>d a basis for concise <strong>de</strong>scription of the polymorphic transformations<br />
occurr<strong>in</strong>g <strong>in</strong> that system. Thus, e.g. transformation of one polymorph<br />
of the acid <strong>in</strong>to another is simply <strong>de</strong>scribed as a conversion of R 2 2(4) <strong>in</strong>to R 2 4(8).<br />
It has been po<strong>in</strong>ted out that graph set analysis of hydrogen bon<strong>de</strong>d systems also<br />
provi<strong>de</strong>s a means for seek<strong>in</strong>g systematic correlations between the resultant<br />
patterns that <strong>de</strong>monstrate “hydrogen bond pattern functionality”[16].This concept<br />
may be useful <strong>in</strong> the prediction of crystal structures as well as for the <strong>de</strong>sign<br />
of materials with <strong>de</strong>sired supramolecular features. Extension of the graph<br />
set approach to encompass <strong>in</strong>termolecular atom-atom <strong>in</strong>teractions other than<br />
hydrogen bond<strong>in</strong>g for the classification of polymorphic crystal structures is<br />
envisaged as a natural and <strong>de</strong>sirable <strong>de</strong>velopment and more wi<strong>de</strong>spread use of<br />
this classification can be expected <strong>in</strong> future.<br />
Elucidation of <strong>de</strong>tailed polymorphic structural features and structural changes<br />
accompany<strong>in</strong>g polymorphic transformations relies heavily on the s<strong>in</strong>gle<br />
crystal X-ray diffraction technique. The <strong>in</strong>ability to produce s<strong>in</strong>gle crystals <strong>in</strong><br />
Fig. 5. Graph set notation for hydrogen bon<strong>de</strong>d r<strong>in</strong>g motifs <strong>in</strong> a polymorph of thalidomi<strong>de</strong>