198 Topics in Current Chemistry Editorial Board: A. de Meijere KN ...
198 Topics in Current Chemistry Editorial Board: A. de Meijere KN ...
198 Topics in Current Chemistry Editorial Board: A. de Meijere KN ...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
84 A. Nangia · G.R. Desiraju<br />
environments. The existence of polymorphic forms may also ren<strong>de</strong>r the molecule<br />
more susceptible to the formation of molecular complexes and this is a<br />
helpful feature. By calculat<strong>in</strong>g the lattice energies of polymorphs, the energies of<br />
molecules <strong>in</strong> different conformations can be estimated. Different supramolecular<br />
synthons <strong>in</strong> polymorphs provi<strong>de</strong> good comparisons of dist<strong>in</strong>ct <strong>in</strong>termolecular<br />
<strong>in</strong>teractions that can arise from the same functional group(s) and their<br />
effects on crystal pack<strong>in</strong>g.<br />
A number of factors have been suggested as possible causes for polymorphism.<br />
Polymorphism can occur when the free energy differences between<br />
alternative crystal structures are very slight. This may happen: (1) because the<br />
<strong>in</strong>termolecular forces are feeble; (2) when the entropic contribution to the free<br />
energy is high; and (3) because k<strong>in</strong>etic rather than thermodynamic factors<br />
control the events lead<strong>in</strong>g to crystallisation. The <strong>de</strong>gree of difference between<br />
polymorphs may itself vary.While there are polymorphs that conta<strong>in</strong> completely<br />
different supramolecular synthons, there are others which conta<strong>in</strong> the same<br />
synthon occurr<strong>in</strong>g <strong>in</strong> slightly different ways. Three dist<strong>in</strong>ct situations are possible:<br />
(1) the same synthons are formed by the same functional groups but the<br />
differences <strong>in</strong> overall pack<strong>in</strong>g are caused by variations <strong>in</strong> the rest of the crystal;<br />
(2) the same synthons are formed by the same functional groups but there are<br />
multiple occurrences of these groups <strong>in</strong> dist<strong>in</strong>ctive molecular locations lead<strong>in</strong>g<br />
to different pack<strong>in</strong>g arrangements; (3) and different synthons are formed<br />
lead<strong>in</strong>g to radically different pack<strong>in</strong>gs. The three situations are discussed with<br />
representative examples [69].<br />
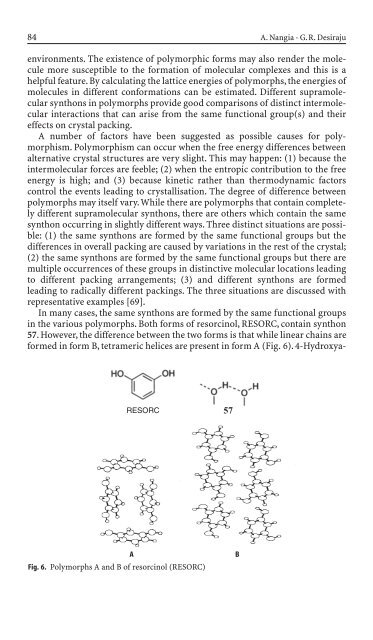
In many cases, the same synthons are formed by the same functional groups<br />
<strong>in</strong> the various polymorphs. Both forms of resorc<strong>in</strong>ol, RESORC, conta<strong>in</strong> synthon<br />
57. However, the difference between the two forms is that while l<strong>in</strong>ear cha<strong>in</strong>s are<br />
formed <strong>in</strong> form B, tetrameric helices are present <strong>in</strong> form A (Fig. 6). 4-Hydroxya-<br />
RESORC 57<br />
A B<br />
Fig. 6. Polymorphs A and B of resorc<strong>in</strong>ol (RESORC)