198 Topics in Current Chemistry Editorial Board: A. de Meijere KN ...
198 Topics in Current Chemistry Editorial Board: A. de Meijere KN ...
198 Topics in Current Chemistry Editorial Board: A. de Meijere KN ...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
Directional Aspects of Intermolecular Interactions 27<br />
group may b<strong>in</strong>d up to six cations and that it may, <strong>in</strong> certa<strong>in</strong> circumstances, share<br />
the metal cation between both oxygen atoms of the carboxylate group <strong>in</strong> a<br />
bi<strong>de</strong>ntate fashion, as has been found for calcium ions [54].<br />
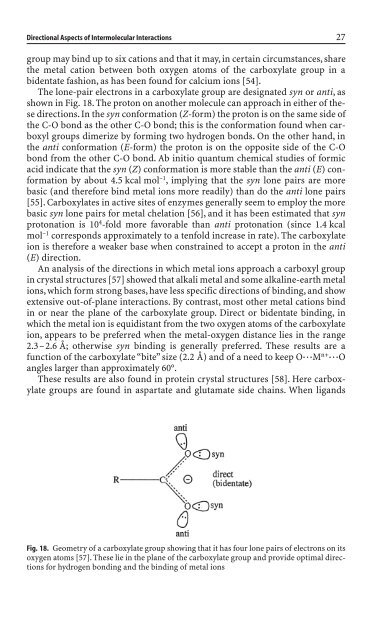
The lone-pair electrons <strong>in</strong> a carboxylate group are <strong>de</strong>signated syn or anti,as<br />
shown <strong>in</strong> Fig. 18. The proton on another molecule can approach <strong>in</strong> either of these<br />
directions. In the syn conformation (Z-form) the proton is on the same si<strong>de</strong> of<br />
the C-O bond as the other C-O bond; this is the conformation found when carboxyl<br />
groups dimerize by form<strong>in</strong>g two hydrogen bonds. On the other hand, <strong>in</strong><br />
the anti conformation (E-form) the proton is on the opposite si<strong>de</strong> of the C-O<br />
bond from the other C-O bond. Ab <strong>in</strong>itio quantum chemical studies of formic<br />
acid <strong>in</strong>dicate that the syn (Z) conformation is more stable than the anti (E) conformation<br />
by about 4.5 kcal mol –1 , imply<strong>in</strong>g that the syn lone pairs are more<br />
basic (and therefore b<strong>in</strong>d metal ions more readily) than do the anti lone pairs<br />
[55]. Carboxylates <strong>in</strong> active sites of enzymes generally seem to employ the more<br />
basic syn lone pairs for metal chelation [56], and it has been estimated that syn<br />
protonation is 10 4 -fold more favorable than anti protonation (s<strong>in</strong>ce 1.4 kcal<br />
mol –1 corresponds approximately to a tenfold <strong>in</strong>crease <strong>in</strong> rate). The carboxylate<br />
ion is therefore a weaker base when constra<strong>in</strong>ed to accept a proton <strong>in</strong> the anti<br />
(E) direction.<br />
An analysis of the directions <strong>in</strong> which metal ions approach a carboxyl group<br />
<strong>in</strong> crystal structures [57] showed that alkali metal and some alkal<strong>in</strong>e-earth metal<br />
ions, which form strong bases, have less specific directions of b<strong>in</strong>d<strong>in</strong>g, and show<br />
extensive out-of-plane <strong>in</strong>teractions. By contrast, most other metal cations b<strong>in</strong>d<br />
<strong>in</strong> or near the plane of the carboxylate group. Direct or bi<strong>de</strong>ntate b<strong>in</strong>d<strong>in</strong>g, <strong>in</strong><br />
which the metal ion is equidistant from the two oxygen atoms of the carboxylate<br />
ion, appears to be preferred when the metal-oxygen distance lies <strong>in</strong> the range<br />
2.3–2.6 Å; otherwise syn b<strong>in</strong>d<strong>in</strong>g is generally preferred. These results are a<br />
function of the carboxylate “bite” size (2.2 Å) and of a need to keep O◊◊◊M n+ ◊◊◊O<br />
angles larger than approximately 60°.<br />
These results are also found <strong>in</strong> prote<strong>in</strong> crystal structures [58]. Here carboxylate<br />
groups are found <strong>in</strong> aspartate and glutamate si<strong>de</strong> cha<strong>in</strong>s. When ligands<br />
Fig. 18. Geometry of a carboxylate group show<strong>in</strong>g that it has four lone pairs of electrons on its<br />
oxygen atoms [57]. These lie <strong>in</strong> the plane of the carboxylate group and provi<strong>de</strong> optimal directions<br />
for hydrogen bond<strong>in</strong>g and the b<strong>in</strong>d<strong>in</strong>g of metal ions