198 Topics in Current Chemistry Editorial Board: A. de Meijere KN ...
198 Topics in Current Chemistry Editorial Board: A. de Meijere KN ...
198 Topics in Current Chemistry Editorial Board: A. de Meijere KN ...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
20 J.P. Glusker<br />
pepti<strong>de</strong> plane and approximately near the C=O bond direction,so that the M◊◊◊O=C<br />
angle is 140–170° for uni<strong>de</strong>ntate b<strong>in</strong>d<strong>in</strong>g and 110–130° for bi<strong>de</strong>ntate b<strong>in</strong>d<strong>in</strong>g. The<br />
metal ion is rarely more than 35° from the pepti<strong>de</strong> plane [36].<br />
4.4<br />
C-H◊◊◊O Interactions<br />
The directionality of a hydrogen bond is partially a function of its strength – the<br />
shorter the hydrogen bond the more likely the A-H◊◊◊B angle is to be near 180°.<br />
Weaker <strong>in</strong>teractions, such as C-H◊◊◊O or C-H◊◊◊N, <strong>de</strong>scribed by Sutor [37], are<br />
found when the hydrogen atom is attached to multiple bonds or strong electron<br />
withdraw<strong>in</strong>g groups (favor<strong>in</strong>g the formation of H + ). These <strong>in</strong>teractions, if<br />
short or the or<strong>de</strong>r of the sum of the van <strong>de</strong>r Waals radii, are generally attractive<br />
rather than repulsive, and have come to be regar<strong>de</strong>d as very weak hydrogen<br />
bonds.<br />
The <strong>in</strong>teraction between a C-H group and a neighbor<strong>in</strong>g oxygen atom has<br />
about one third the energy of an O-H◊◊◊O hydrogen bond. If, however, there is a<br />
large number of such <strong>in</strong>teractions, their contributions to the total energy of a<br />
system may be substantial. For example, the importance of C-H◊◊◊O <strong>in</strong>teractions<br />
<strong>in</strong> align<strong>in</strong>g molecules has been shown by the <strong>de</strong>sign, by Desiraju and coworkers,<br />
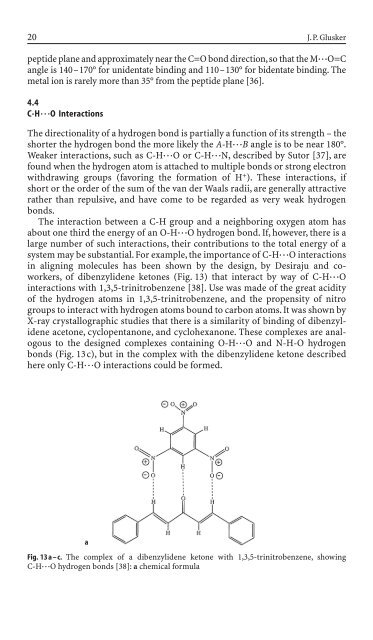
of dibenzyli<strong>de</strong>ne ketones (Fig. 13) that <strong>in</strong>teract by way of C-H◊◊◊O<br />
<strong>in</strong>teractions with 1,3,5-tr<strong>in</strong>itrobenzene [38]. Use was ma<strong>de</strong> of the great acidity<br />
of the hydrogen atoms <strong>in</strong> 1,3,5-tr<strong>in</strong>itrobenzene, and the propensity of nitro<br />
groups to <strong>in</strong>teract with hydrogen atoms bound to carbon atoms. It was shown by<br />
X-ray crystallographic studies that there is a similarity of b<strong>in</strong>d<strong>in</strong>g of dibenzyli<strong>de</strong>ne<br />
acetone, cyclopentanone, and cyclohexanone. These complexes are analogous<br />
to the <strong>de</strong>signed complexes conta<strong>in</strong><strong>in</strong>g O-H◊◊◊O and N-H-O hydrogen<br />
bonds (Fig. 13c), but <strong>in</strong> the complex with the dibenzyli<strong>de</strong>ne ketone <strong>de</strong>scribed<br />
here only C-H◊◊◊O <strong>in</strong>teractions could be formed.<br />
a<br />
Fig. 13 a – c. The complex of a dibenzyli<strong>de</strong>ne ketone with 1,3,5-tr<strong>in</strong>itrobenzene, show<strong>in</strong>g<br />
C-H◊◊◊O hydrogen bonds [38]: a chemical formula