198 Topics in Current Chemistry Editorial Board: A. de Meijere KN ...
198 Topics in Current Chemistry Editorial Board: A. de Meijere KN ...
198 Topics in Current Chemistry Editorial Board: A. de Meijere KN ...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
72 A. Nangia · G.R. Desiraju<br />
<strong>in</strong>gful. This is because: (1) the crystal structure of any molecule must have a few<br />
<strong>in</strong>termolecular contacts <strong>in</strong> the repulsive van <strong>de</strong>r Waals region – a few such forced<br />
contacts can be comfortably accommodated <strong>in</strong> any crystal structure provi<strong>de</strong>d<br />
they are compensated for by the free energy ga<strong>in</strong>ed from the other numerous<br />
dispersive forces, hydrogen bonds and heteroatom <strong>in</strong>teractions; (2) the<br />
weak, polarisable hydrogen bonds (C-H◊◊◊O, C-H◊◊◊N, C-H◊◊◊X, O-H◊◊◊p) and<br />
other heteroatom <strong>in</strong>teractions (X◊◊◊X, N◊◊◊X, S◊◊◊X) have approach distances and<br />
angle characteristics scattered over a wi<strong>de</strong>r range – they are also more susceptible<br />
to the mutually <strong>in</strong>terfer<strong>in</strong>g effects <strong>in</strong> a crystal, effects that can <strong>de</strong>form consi<strong>de</strong>rably<br />
the approach vector from the i<strong>de</strong>al geometry.<br />
In the statistical approach, such as is possible with the CSD, a large number of<br />
observed <strong>in</strong>teraction geometries cluster <strong>in</strong> a narrow region. A few outliers are<br />
<strong>de</strong>formed and may be repulsive <strong>in</strong> nature. The larger the number of data po<strong>in</strong>ts,<br />
the greater is the confi<strong>de</strong>nce <strong>in</strong> the <strong>in</strong>termolecular contact or structural hypothesis<br />
un<strong>de</strong>r study. As for the outliers, they could furnish an additional bonus <strong>in</strong><br />
that their occurrence is often <strong>in</strong>dicative of an unusual or different chemical<br />
effect.<br />
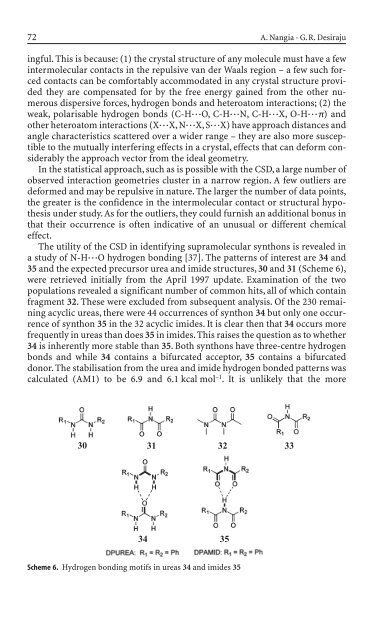
The utility of the CSD <strong>in</strong> i<strong>de</strong>ntify<strong>in</strong>g supramolecular synthons is revealed <strong>in</strong><br />
a study of N-H◊◊◊O hydrogen bond<strong>in</strong>g [37]. The patterns of <strong>in</strong>terest are 34 and<br />
35 and the expected precursor urea and imi<strong>de</strong> structures, 30 and 31 (Scheme 6),<br />
were retrieved <strong>in</strong>itially from the April 1997 update. Exam<strong>in</strong>ation of the two<br />
populations revealed a significant number of common hits, all of which conta<strong>in</strong><br />
fragment 32. These were exclu<strong>de</strong>d from subsequent analysis. Of the 230 rema<strong>in</strong><strong>in</strong>g<br />
acyclic ureas, there were 44 occurrences of synthon 34 but only one occurrence<br />
of synthon 35 <strong>in</strong> the 32 acyclic imi<strong>de</strong>s. It is clear then that 34 occurs more<br />
frequently <strong>in</strong> ureas than does 35 <strong>in</strong> imi<strong>de</strong>s. This raises the question as to whether<br />
34 is <strong>in</strong>herently more stable than 35. Both synthons have three-centre hydrogen<br />
bonds and while 34 conta<strong>in</strong>s a bifurcated acceptor, 35 conta<strong>in</strong>s a bifurcated<br />
donor. The stabilisation from the urea and imi<strong>de</strong> hydrogen bon<strong>de</strong>d patterns was<br />
calculated (AM1) to be 6.9 and 6.1 kcal mol –1 . It is unlikely that the more<br />
30 31 32 33<br />
34 35<br />
Scheme 6. Hydrogen bond<strong>in</strong>g motifs <strong>in</strong> ureas 34 and imi<strong>de</strong>s 35