198 Topics in Current Chemistry Editorial Board: A. de Meijere KN ...
198 Topics in Current Chemistry Editorial Board: A. de Meijere KN ...
198 Topics in Current Chemistry Editorial Board: A. de Meijere KN ...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
Crystall<strong>in</strong>e Polymorphism of Organic Compounds 167<br />
a<br />
b c<br />
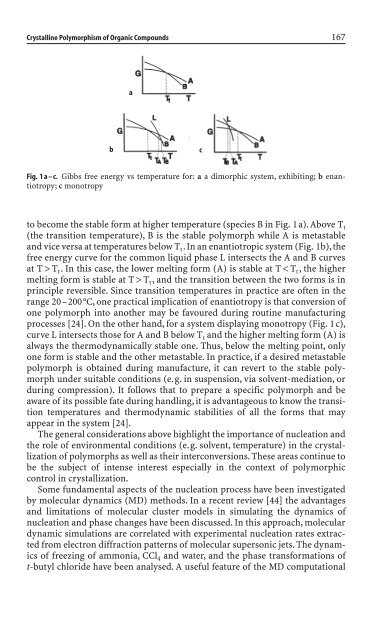
Fig. 1a–c. Gibbs free energy vs temperature for: a a dimorphic system, exhibit<strong>in</strong>g; b enantiotropy;<br />
c monotropy<br />
to become the stable form at higher temperature (species B <strong>in</strong> Fig. 1a). Above T t<br />
(the transition temperature), B is the stable polymorph while A is metastable<br />
and vice versa at temperatures below T t.In an enantiotropic system (Fig. 1b),the<br />
free energy curve for the common liquid phase L <strong>in</strong>tersects the A and B curves<br />
at T > T t. In this case, the lower melt<strong>in</strong>g form (A) is stable at T < T t, the higher<br />
melt<strong>in</strong>g form is stable at T > T t, and the transition between the two forms is <strong>in</strong><br />
pr<strong>in</strong>ciple reversible. S<strong>in</strong>ce transition temperatures <strong>in</strong> practice are often <strong>in</strong> the<br />
range 20–200°C, one practical implication of enantiotropy is that conversion of<br />
one polymorph <strong>in</strong>to another may be favoured dur<strong>in</strong>g rout<strong>in</strong>e manufactur<strong>in</strong>g<br />
processes [24]. On the other hand, for a system display<strong>in</strong>g monotropy (Fig. 1c),<br />
curve L <strong>in</strong>tersects those for A and B below T t and the higher melt<strong>in</strong>g form (A) is<br />
always the thermodynamically stable one. Thus, below the melt<strong>in</strong>g po<strong>in</strong>t, only<br />
one form is stable and the other metastable. In practice, if a <strong>de</strong>sired metastable<br />
polymorph is obta<strong>in</strong>ed dur<strong>in</strong>g manufacture, it can revert to the stable polymorph<br />
un<strong>de</strong>r suitable conditions (e.g. <strong>in</strong> suspension, via solvent-mediation, or<br />
dur<strong>in</strong>g compression). It follows that to prepare a specific polymorph and be<br />
aware of its possible fate dur<strong>in</strong>g handl<strong>in</strong>g, it is advantageous to know the transition<br />
temperatures and thermodynamic stabilities of all the forms that may<br />
appear <strong>in</strong> the system [24].<br />
The general consi<strong>de</strong>rations above highlight the importance of nucleation and<br />
the role of environmental conditions (e.g. solvent, temperature) <strong>in</strong> the crystallization<br />
of polymorphs as well as their <strong>in</strong>terconversions. These areas cont<strong>in</strong>ue to<br />
be the subject of <strong>in</strong>tense <strong>in</strong>terest especially <strong>in</strong> the context of polymorphic<br />
control <strong>in</strong> crystallization.<br />
Some fundamental aspects of the nucleation process have been <strong>in</strong>vestigated<br />
by molecular dynamics (MD) methods. In a recent review [44] the advantages<br />
and limitations of molecular cluster mo<strong>de</strong>ls <strong>in</strong> simulat<strong>in</strong>g the dynamics of<br />
nucleation and phase changes have been discussed. In this approach, molecular<br />
dynamic simulations are correlated with experimental nucleation rates extracted<br />
from electron diffraction patterns of molecular supersonic jets. The dynamics<br />
of freez<strong>in</strong>g of ammonia, CCl 4 and water, and the phase transformations of<br />
t-butyl chlori<strong>de</strong> have been analysed. A useful feature of the MD computational