198 Topics in Current Chemistry Editorial Board: A. de Meijere KN ...
198 Topics in Current Chemistry Editorial Board: A. de Meijere KN ...
198 Topics in Current Chemistry Editorial Board: A. de Meijere KN ...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
Supramolecular Synthons and Pattern Recognition 85<br />
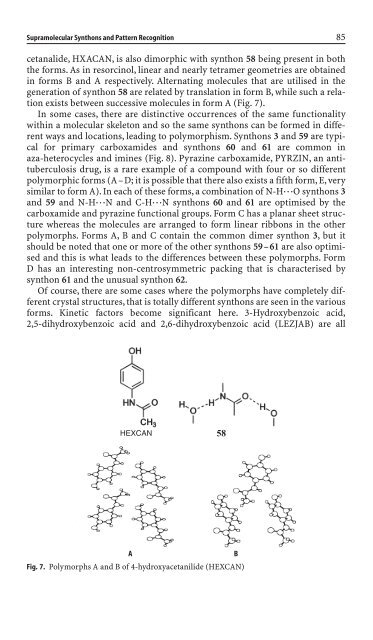
cetanali<strong>de</strong>, HXACAN, is also dimorphic with synthon 58 be<strong>in</strong>g present <strong>in</strong> both<br />
the forms. As <strong>in</strong> resorc<strong>in</strong>ol, l<strong>in</strong>ear and nearly tetramer geometries are obta<strong>in</strong>ed<br />
<strong>in</strong> forms B and A respectively. Alternat<strong>in</strong>g molecules that are utilised <strong>in</strong> the<br />
generation of synthon 58 are related by translation <strong>in</strong> form B, while such a relation<br />
exists between successive molecules <strong>in</strong> form A (Fig. 7).<br />
In some cases, there are dist<strong>in</strong>ctive occurrences of the same functionality<br />
with<strong>in</strong> a molecular skeleton and so the same synthons can be formed <strong>in</strong> different<br />
ways and locations, lead<strong>in</strong>g to polymorphism. Synthons 3 and 59 are typical<br />
for primary carboxami<strong>de</strong>s and synthons 60 and 61 are common <strong>in</strong><br />
aza-heterocycles and im<strong>in</strong>es (Fig. 8). Pyraz<strong>in</strong>e carboxami<strong>de</strong>, PYRZIN, an antituberculosis<br />
drug, is a rare example of a compound with four or so different<br />
polymorphic forms (A–D; it is possible that there also exists a fifth form, E, very<br />
similar to form A). In each of these forms, a comb<strong>in</strong>ation of N-H◊◊◊O synthons 3<br />
and 59 and N-H◊◊◊N and C-H◊◊◊N synthons 60 and 61 are optimised by the<br />
carboxami<strong>de</strong> and pyraz<strong>in</strong>e functional groups. Form C has a planar sheet structure<br />
whereas the molecules are arranged to form l<strong>in</strong>ear ribbons <strong>in</strong> the other<br />
polymorphs. Forms A, B and C conta<strong>in</strong> the common dimer synthon 3, but it<br />
should be noted that one or more of the other synthons 59–61 are also optimised<br />
and this is what leads to the differences between these polymorphs. Form<br />
D has an <strong>in</strong>terest<strong>in</strong>g non-centrosymmetric pack<strong>in</strong>g that is characterised by<br />
synthon 61 and the unusual synthon 62.<br />
Of course, there are some cases where the polymorphs have completely different<br />
crystal structures, that is totally different synthons are seen <strong>in</strong> the various<br />
forms. K<strong>in</strong>etic factors become significant here. 3-Hydroxybenzoic acid,<br />
2,5-dihydroxybenzoic acid and 2,6-dihydroxybenzoic acid (LEZJAB) are all<br />
HEXCAN 58<br />
A B<br />
Fig. 7. Polymorphs A and B of 4-hydroxyacetanili<strong>de</strong> (HEXCAN)