198 Topics in Current Chemistry Editorial Board: A. de Meijere KN ...
198 Topics in Current Chemistry Editorial Board: A. de Meijere KN ...
198 Topics in Current Chemistry Editorial Board: A. de Meijere KN ...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
80 A. Nangia · G.R. Desiraju<br />
from nonpolar regions of the macromolecule. It is well known that the difference<br />
between a molecule that b<strong>in</strong>ds to the active site with m<strong>in</strong>imal si<strong>de</strong> effects, and<br />
hence a potential drug candidate, and the numerous ligands that do not is usually<br />
a very small structural difference <strong>in</strong> atom type, position or stereochemistry.<br />
The objective of rational drug <strong>de</strong>sign is therefore to predict new molecules or<br />
analogues of known drugs which will have favourable <strong>in</strong>teractions with a prote<strong>in</strong><br />
of known structure [63, 64]. The three crucial <strong>in</strong>puts for structure-based<br />
ligand <strong>de</strong>sign are: (1) the molecular structure of the ligand; (2) the shape of the<br />
macromolecular receptor prote<strong>in</strong>; and (3) the typical <strong>in</strong>termolecular <strong>in</strong>teraction<br />
patterns between ligand and receptor. The last of these is with<strong>in</strong> the scope of this<br />
article and is discussed here.<br />
The number of accurate crystallographically resolved ligand-prote<strong>in</strong> structures<br />
is still not large and hence a systematic, statistical analysis of their <strong>in</strong>teraction<br />
geometry is not possible. However, the enormous wealth of <strong>in</strong>formation<br />
on small molecule crystallography <strong>in</strong> the CSD can be retrieved to extract orientational<br />
preferences for <strong>in</strong>teraction around different functional groups. Klebe<br />
has presented composite crystal field environments for the approach of different<br />
donor hydrogens to various acceptor atoms,<strong>in</strong> effect compil<strong>in</strong>g a hydrogen bond<br />
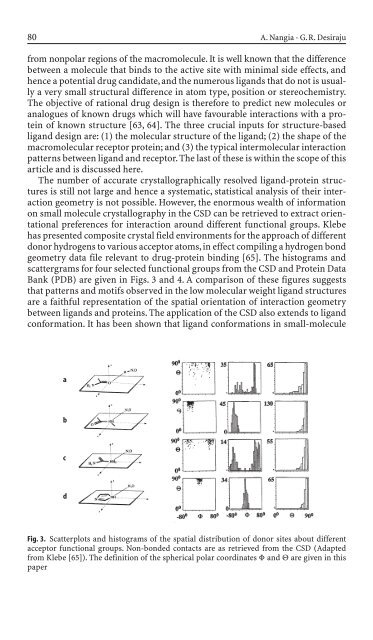
geometry data file relevant to drug-prote<strong>in</strong> b<strong>in</strong>d<strong>in</strong>g [65]. The histograms and<br />
scattergrams for four selected functional groups from the CSD and Prote<strong>in</strong> Data<br />
Bank (PDB) are given <strong>in</strong> Figs. 3 and 4. A comparison of these figures suggests<br />
that patterns and motifs observed <strong>in</strong> the low molecular weight ligand structures<br />
are a faithful representation of the spatial orientation of <strong>in</strong>teraction geometry<br />
between ligands and prote<strong>in</strong>s. The application of the CSD also extends to ligand<br />
conformation. It has been shown that ligand conformations <strong>in</strong> small-molecule<br />
a<br />
b<br />
c<br />
d<br />
Fig. 3. Scatterplots and histograms of the spatial distribution of donor sites about different<br />
acceptor functional groups. Non-bon<strong>de</strong>d contacts are as retrieved from the CSD (Adapted<br />
from Klebe [65]). The <strong>de</strong>f<strong>in</strong>ition of the spherical polar coord<strong>in</strong>ates F and Q are given <strong>in</strong> this<br />
paper