198 Topics in Current Chemistry Editorial Board: A. de Meijere KN ...
198 Topics in Current Chemistry Editorial Board: A. de Meijere KN ...
198 Topics in Current Chemistry Editorial Board: A. de Meijere KN ...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
Directional Aspects of Intermolecular Interactions 13<br />
c<br />
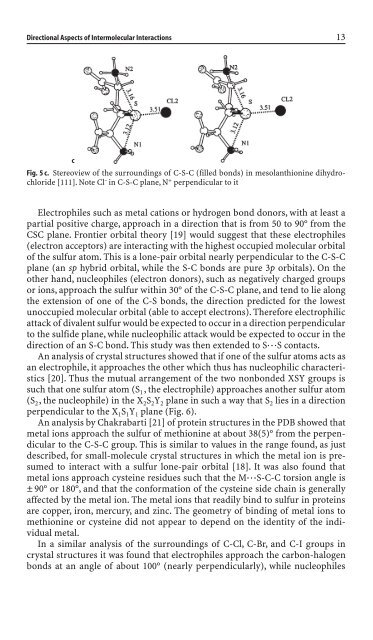
Fig. 5 c. Stereoview of the surround<strong>in</strong>gs of C-S-C (filled bonds) <strong>in</strong> mesolanthion<strong>in</strong>e dihydrochlori<strong>de</strong><br />
[111]. Note Cl – <strong>in</strong> C-S-C plane, N + perpendicular to it<br />
Electrophiles such as metal cations or hydrogen bond donors, with at least a<br />
partial positive charge, approach <strong>in</strong> a direction that is from 50 to 90° from the<br />
CSC plane. Frontier orbital theory [19] would suggest that these electrophiles<br />
(electron acceptors) are <strong>in</strong>teract<strong>in</strong>g with the highest occupied molecular orbital<br />
of the sulfur atom. This is a lone-pair orbital nearly perpendicular to the C-S-C<br />
plane (an sp hybrid orbital, while the S-C bonds are pure 3p orbitals). On the<br />
other hand, nucleophiles (electron donors), such as negatively charged groups<br />
or ions, approach the sulfur with<strong>in</strong> 30° of the C-S-C plane, and tend to lie along<br />
the extension of one of the C-S bonds, the direction predicted for the lowest<br />
unoccupied molecular orbital (able to accept electrons). Therefore electrophilic<br />
attack of divalent sulfur would be expected to occur <strong>in</strong> a direction perpendicular<br />
to the sulfi<strong>de</strong> plane, while nucleophilic attack would be expected to occur <strong>in</strong> the<br />
direction of an S-C bond. This study was then exten<strong>de</strong>d to S◊◊◊S contacts.<br />
An analysis of crystal structures showed that if one of the sulfur atoms acts as<br />
an electrophile, it approaches the other which thus has nucleophilic characteristics<br />
[20]. Thus the mutual arrangement of the two nonbon<strong>de</strong>d XSY groups is<br />
such that one sulfur atom (S 1, the electrophile) approaches another sulfur atom<br />
(S 2, the nucleophile) <strong>in</strong> the X 2S 2Y 2 plane <strong>in</strong> such a way that S 2 lies <strong>in</strong> a direction<br />
perpendicular to the X 1S 1Y 1 plane (Fig. 6).<br />
An analysis by Chakrabarti [21] of prote<strong>in</strong> structures <strong>in</strong> the PDB showed that<br />
metal ions approach the sulfur of methion<strong>in</strong>e at about 38(5)° from the perpendicular<br />
to the C-S-C group. This is similar to values <strong>in</strong> the range found, as just<br />
<strong>de</strong>scribed, for small-molecule crystal structures <strong>in</strong> which the metal ion is presumed<br />
to <strong>in</strong>teract with a sulfur lone-pair orbital [18]. It was also found that<br />
metal ions approach cyste<strong>in</strong>e residues such that the M◊◊◊S-C-C torsion angle is<br />
± 90° or 180°, and that the conformation of the cyste<strong>in</strong>e si<strong>de</strong> cha<strong>in</strong> is generally<br />
affected by the metal ion. The metal ions that readily b<strong>in</strong>d to sulfur <strong>in</strong> prote<strong>in</strong>s<br />
are copper, iron, mercury, and z<strong>in</strong>c. The geometry of b<strong>in</strong>d<strong>in</strong>g of metal ions to<br />
methion<strong>in</strong>e or cyste<strong>in</strong>e did not appear to <strong>de</strong>pend on the i<strong>de</strong>ntity of the <strong>in</strong>dividual<br />
metal.<br />
In a similar analysis of the surround<strong>in</strong>gs of C-Cl, C-Br, and C-I groups <strong>in</strong><br />
crystal structures it was found that electrophiles approach the carbon-halogen<br />
bonds at an angle of about 100° (nearly perpendicularly), while nucleophiles