198 Topics in Current Chemistry Editorial Board: A. de Meijere KN ...
198 Topics in Current Chemistry Editorial Board: A. de Meijere KN ...
198 Topics in Current Chemistry Editorial Board: A. de Meijere KN ...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
148 Y. Aoyama<br />
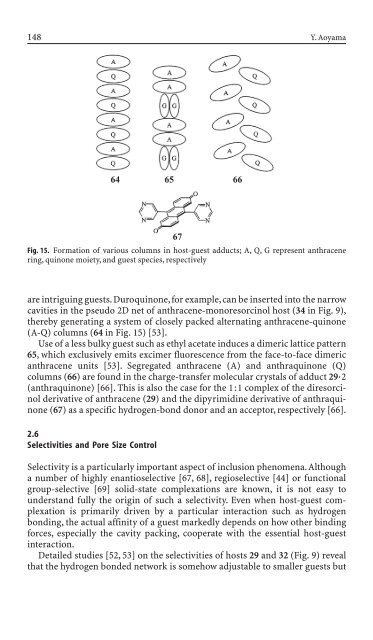
are <strong>in</strong>trigu<strong>in</strong>g guests. Duroqu<strong>in</strong>one, for example, can be <strong>in</strong>serted <strong>in</strong>to the narrow<br />
cavities <strong>in</strong> the pseudo 2D net of anthracene-monoresorc<strong>in</strong>ol host (34 <strong>in</strong> Fig. 9),<br />
thereby generat<strong>in</strong>g a system of closely packed alternat<strong>in</strong>g anthracene-qu<strong>in</strong>one<br />
(A-Q) columns (64 <strong>in</strong> Fig. 15) [53].<br />
Use of a less bulky guest such as ethyl acetate <strong>in</strong>duces a dimeric lattice pattern<br />
65, which exclusively emits excimer fluorescence from the face-to-face dimeric<br />
anthracene units [53]. Segregated anthracene (A) and anthraqu<strong>in</strong>one (Q)<br />
columns (66) are found <strong>in</strong> the charge-transfer molecular crystals of adduct 29◊2<br />
(anthraqu<strong>in</strong>one) [66]. This is also the case for the 1:1 complex of the diresorc<strong>in</strong>ol<br />
<strong>de</strong>rivative of anthracene (29) and the dipyrimid<strong>in</strong>e <strong>de</strong>rivative of anthraqu<strong>in</strong>one<br />
(67) as a specific hydrogen-bond donor and an acceptor, respectively [66].<br />
2.6<br />
Selectivities and Pore Size Control<br />
64 65 66<br />
67<br />
Fig. 15. Formation of various columns <strong>in</strong> host-guest adducts; A, Q, G represent anthracene<br />
r<strong>in</strong>g, qu<strong>in</strong>one moiety, and guest species, respectively<br />
Selectivity is a particularly important aspect of <strong>in</strong>clusion phenomena.Although<br />
a number of highly enantioselective [67, 68], regioselective [44] or functional<br />
group-selective [69] solid-state complexations are known, it is not easy to<br />
un<strong>de</strong>rstand fully the orig<strong>in</strong> of such a selectivity. Even when host-guest complexation<br />
is primarily driven by a particular <strong>in</strong>teraction such as hydrogen<br />
bond<strong>in</strong>g, the actual aff<strong>in</strong>ity of a guest markedly <strong>de</strong>pends on how other b<strong>in</strong>d<strong>in</strong>g<br />
forces, especially the cavity pack<strong>in</strong>g, cooperate with the essential host-guest<br />
<strong>in</strong>teraction.<br />
Detailed studies [52, 53] on the selectivities of hosts 29 and 32 (Fig. 9) reveal<br />
that the hydrogen bon<strong>de</strong>d network is somehow adjustable to smaller guests but