198 Topics in Current Chemistry Editorial Board: A. de Meijere KN ...
198 Topics in Current Chemistry Editorial Board: A. de Meijere KN ...
198 Topics in Current Chemistry Editorial Board: A. de Meijere KN ...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
186 M.R. Caira<br />
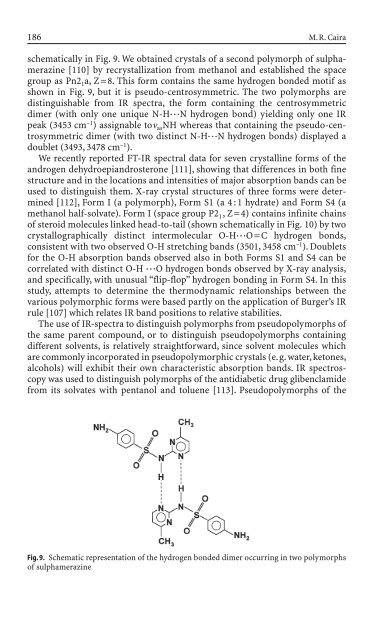
schematically <strong>in</strong> Fig. 9. We obta<strong>in</strong>ed crystals of a second polymorph of sulphameraz<strong>in</strong>e<br />
[110] by recrystallization from methanol and established the space<br />
group as Pn2 1a, Z=8. This form conta<strong>in</strong>s the same hydrogen bon<strong>de</strong>d motif as<br />
shown <strong>in</strong> Fig. 9, but it is pseudo-centrosymmetric. The two polymorphs are<br />
dist<strong>in</strong>guishable from IR spectra, the form conta<strong>in</strong><strong>in</strong>g the centrosymmetric<br />
dimer (with only one unique N-H◊◊◊N hydrogen bond) yield<strong>in</strong>g only one IR<br />
peak (3453 cm –1 ) assignable ton asNH whereas that conta<strong>in</strong><strong>in</strong>g the pseudo-centrosymmetric<br />
dimer (with two dist<strong>in</strong>ct N-H◊◊◊N hydrogen bonds) displayed a<br />
doublet (3493, 3478 cm –1 ).<br />
We recently reported FT-IR spectral data for seven crystall<strong>in</strong>e forms of the<br />
androgen <strong>de</strong>hydroepiandrosterone [111], show<strong>in</strong>g that differences <strong>in</strong> both f<strong>in</strong>e<br />
structure and <strong>in</strong> the locations and <strong>in</strong>tensities of major absorption bands can be<br />
used to dist<strong>in</strong>guish them. X-ray crystal structures of three forms were <strong>de</strong>term<strong>in</strong>ed<br />
[112], Form I (a polymorph), Form S1 (a 4 : 1 hydrate) and Form S4 (a<br />
methanol half-solvate). Form I (space group P2 1, Z=4) conta<strong>in</strong>s <strong>in</strong>f<strong>in</strong>ite cha<strong>in</strong>s<br />
of steroid molecules l<strong>in</strong>ked head-to-tail (shown schematically <strong>in</strong> Fig. 10) by two<br />
crystallographically dist<strong>in</strong>ct <strong>in</strong>termolecular O-H◊◊◊O=C hydrogen bonds,<br />
consistent with two observed O-H stretch<strong>in</strong>g bands (3501, 3458 cm –1 ). Doublets<br />
for the O-H absorption bands observed also <strong>in</strong> both Forms S1 and S4 can be<br />
correlated with dist<strong>in</strong>ct O-H ◊◊◊O hydrogen bonds observed by X-ray analysis,<br />
and specifically, with unusual “flip-flop” hydrogen bond<strong>in</strong>g <strong>in</strong> Form S4. In this<br />
study, attempts to <strong>de</strong>term<strong>in</strong>e the thermodynamic relationships between the<br />
various polymorphic forms were based partly on the application of Burger’s IR<br />
rule [107] which relates IR band positions to relative stabilities.<br />
The use of IR-spectra to dist<strong>in</strong>guish polymorphs from pseudopolymorphs of<br />
the same parent compound, or to dist<strong>in</strong>guish pseudopolymorphs conta<strong>in</strong><strong>in</strong>g<br />
different solvents, is relatively straightforward, s<strong>in</strong>ce solvent molecules which<br />
are commonly <strong>in</strong>corporated <strong>in</strong> pseudopolymorphic crystals (e.g. water, ketones,<br />
alcohols) will exhibit their own characteristic absorption bands. IR spectroscopy<br />
was used to dist<strong>in</strong>guish polymorphs of the antidiabetic drug glibenclami<strong>de</strong><br />
from its solvates with pentanol and toluene [113]. Pseudopolymorphs of the<br />
Fig. 9. Schematic representation of the hydrogen bon<strong>de</strong>d dimer occurr<strong>in</strong>g <strong>in</strong> two polymorphs<br />
of sulphameraz<strong>in</strong>e