Monograph on the Potential Human Reproductive and ... - OEHHA
Monograph on the Potential Human Reproductive and ... - OEHHA
Monograph on the Potential Human Reproductive and ... - OEHHA
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
220 CHAPIN ET AL.<br />
Table 54<br />
Differences Between Laboratories in Rat Uterotrophic<br />
Assay With Bisphenol A a<br />
Laboratory Rat strain<br />
Immature,<br />
gavage x 3 days<br />
2<br />
7<br />
12<br />
13<br />
Immature,<br />
s.c. x 3 days<br />
2<br />
6<br />
7<br />
8<br />
12<br />
13<br />
15<br />
18<br />
20<br />
21<br />
Adult, s.c.<br />
x 3 days<br />
2<br />
6<br />
7<br />
8<br />
12<br />
Adult, s.c.<br />
x 7 days<br />
2<br />
7<br />
a Kanno et al. (2003b).<br />
CD(SD)IGS<br />
CD(SD)IGS<br />
CD(SD)IGS BR<br />
Wistar<br />
CD(SD)IGS<br />
CD(SD)IGS BR<br />
CD(SD)IGS<br />
Alpk:ApfSD<br />
CD(SD)IGS BR<br />
Wistar<br />
Wistar<br />
Sprague–Dawley<br />
Sprague–Dawley<br />
CD(SD) BR<br />
CD(SD)IGS<br />
CD(SD)IGS BR<br />
CD(SD)IGS<br />
Alpk:ApfSD<br />
CD(SD)IGS BR<br />
CD(SD)IGS<br />
CD(SD)IGS<br />
Lowest effective dose<br />
level (mg/kg bw/day)<br />
200<br />
10<br />
x<br />
x<br />
x<br />
10<br />
10<br />
375<br />
x<br />
x<br />
100<br />
x<br />
x<br />
x<br />
100<br />
x<br />
x<br />
x<br />
x<br />
x<br />
100<br />
x<br />
x<br />
600<br />
x<br />
300<br />
x<br />
x<br />
x<br />
300<br />
300<br />
1000<br />
x<br />
600<br />
x<br />
600<br />
600<br />
1000<br />
1000<br />
1000<br />
in juvenile ovariectomized DA/Han, Sprague–Dawley,<br />
<strong>and</strong> Wistar rats. After 3 days of treatment with bisphenol<br />
A 200 mg/kg bw/day, <strong>the</strong>re were small statistically<br />
significant increases in uterine weight in DA/Han <strong>and</strong><br />
Sprague–Dawley rats but not in Wistar rats. There were<br />
no alterati<strong>on</strong>s in uterine or vaginal epi<strong>the</strong>lium or in<br />
uterine clusterin mRNA expressi<strong>on</strong> in any of <strong>the</strong> strains<br />
after bisphenol A treatment.<br />
Inter-laboratory variati<strong>on</strong> in <strong>the</strong> uterotrophic assay<br />
was evaluated by <strong>the</strong> Organizati<strong>on</strong> for Ec<strong>on</strong>omic<br />
Cooperati<strong>on</strong> <strong>and</strong> Development (OECD) (Kanno et al.,<br />
2003b). Coded chemicals, including bisphenol A, were<br />
sent to up to 212 different laboratories. Four assay<br />
protocols were evaluated including oral treatment of<br />
intact immature rats for 3 days, s.c. treatment of intact<br />
immature rats for 3 days, s.c. treatment of ovariectomized<br />
6–8-week-old rats for 3 days, <strong>and</strong> s.c. treatment of<br />
ovariectomized 6–8-week-old rats for 7 days. Not all<br />
laboratories used all protocols or tested all compounds.<br />
Rat strains <strong>and</strong> suppliers were not st<strong>and</strong>ardized across<br />
laboratories. Comparis<strong>on</strong>s were made between labs<br />
based <strong>on</strong> <strong>the</strong> lowest dose level at which body weightadjusted<br />
blotted uterine weight was significantly different<br />
from <strong>the</strong> c<strong>on</strong>trol. Results are summarized in Table 54.<br />
The lowest effective dose of bisphenol A was uniformly<br />
identified for <strong>the</strong> assays performed in ovariectomized<br />
adults. Assays performed in immature animals varied in<br />
identificati<strong>on</strong> of <strong>the</strong> lowest effective bisphenol A dose<br />
Multiple of c<strong>on</strong>trol value<br />
6.0<br />
5.5<br />
5.0<br />
PCNA labeling<br />
4.5<br />
3.0<br />
2.5 Vaginal opening PND 26<br />
2.0<br />
Epi<strong>the</strong>lial cell height<br />
1.5 Uterine wet weight<br />
1.0<br />
Body weight<br />
0.5<br />
0.0<br />
0.1 1.0 10.0 100.0<br />
Bisphenol A dose, mg/kg bw/day<br />
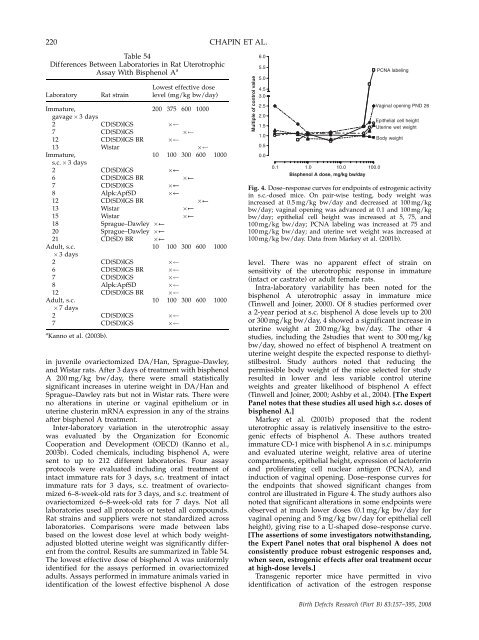
Fig. 4. Dose–resp<strong>on</strong>se curves for endpoints of estrogenic activity<br />
in s.c.-dosed mice. On pair-wise testing, body weight was<br />
increased at 0.5 mg/kg bw/day <strong>and</strong> decreased at 100 mg/kg<br />
bw/day; vaginal opening was advanced at 0.1 <strong>and</strong> 100 mg/kg<br />
bw/day; epi<strong>the</strong>lial cell height was increased at 5, 75, <strong>and</strong><br />
100 mg/kg bw/day; PCNA labeling was increased at 75 <strong>and</strong><br />
100 mg/kg bw/day; <strong>and</strong> uterine wet weight was increased at<br />
100 mg/kg bw/day. Data from Markey et al. (2001b).<br />
level. There was no apparent effect of strain <strong>on</strong><br />
sensitivity of <strong>the</strong> uterotrophic resp<strong>on</strong>se in immature<br />
(intact or castrate) or adult female rats.<br />
Intra-laboratory variability has been noted for <strong>the</strong><br />
bisphenol A uterotrophic assay in immature mice<br />
(Tinwell <strong>and</strong> Joiner, 2000). Of 8 studies performed over<br />
a 2-year period at s.c. bisphenol A dose levels up to 200<br />
or 300 mg/kg bw/day, 4 showed a significant increase in<br />
uterine weight at 200 mg/kg bw/day. The o<strong>the</strong>r 4<br />
studies, including <strong>the</strong> 2studies that went to 300 mg/kg<br />
bw/day, showed no effect of bisphenol A treatment <strong>on</strong><br />
uterine weight despite <strong>the</strong> expected resp<strong>on</strong>se to diethylstilbestrol.<br />
Study authors noted that reducing <strong>the</strong><br />
permissible body weight of <strong>the</strong> mice selected for study<br />
resulted in lower <strong>and</strong> less variable c<strong>on</strong>trol uterine<br />
weights <strong>and</strong> greater likelihood of bisphenol A effect<br />
(Tinwell <strong>and</strong> Joiner, 2000; Ashby et al., 2004). [The Expert<br />
Panel notes that <strong>the</strong>se studies all used high s.c. doses of<br />
bisphenol A.]<br />
Markey et al. (2001b) proposed that <strong>the</strong> rodent<br />
uterotrophic assay is relatively insensitive to <strong>the</strong> estrogenic<br />
effects of bisphenol A. These authors treated<br />
immature CD-1 mice with bisphenol A in s.c. minipumps<br />
<strong>and</strong> evaluated uterine weight, relative area of uterine<br />
compartments, epi<strong>the</strong>lial height, expressi<strong>on</strong> of lactoferrin<br />
<strong>and</strong> proliferating cell nuclear antigen (PCNA), <strong>and</strong><br />
inducti<strong>on</strong> of vaginal opening. Dose–resp<strong>on</strong>se curves for<br />
<strong>the</strong> endpoints that showed significant changes from<br />
c<strong>on</strong>trol are illustrated in Figure 4. The study authors also<br />
noted that significant alterati<strong>on</strong>s in some endpoints were<br />
observed at much lower doses (0.1 mg/kg bw/day for<br />
vaginal opening <strong>and</strong> 5 mg/kg bw/day for epi<strong>the</strong>lial cell<br />
height), giving rise to a U-shaped dose–resp<strong>on</strong>se curve.<br />
[The asserti<strong>on</strong>s of some investigators notwithst<strong>and</strong>ing,<br />
<strong>the</strong> Expert Panel notes that oral bisphenol A does not<br />
c<strong>on</strong>sistently produce robust estrogenic resp<strong>on</strong>ses <strong>and</strong>,<br />
when seen, estrogenic effects after oral treatment occur<br />
at high-dose levels.]<br />
Transgenic reporter mice have permitted in vivo<br />
identificati<strong>on</strong> of activati<strong>on</strong> of <strong>the</strong> estrogen resp<strong>on</strong>se<br />
Birth Defects Research (Part B) 83:157–395, 2008