Monograph on the Potential Human Reproductive and ... - OEHHA
Monograph on the Potential Human Reproductive and ... - OEHHA
Monograph on the Potential Human Reproductive and ... - OEHHA
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
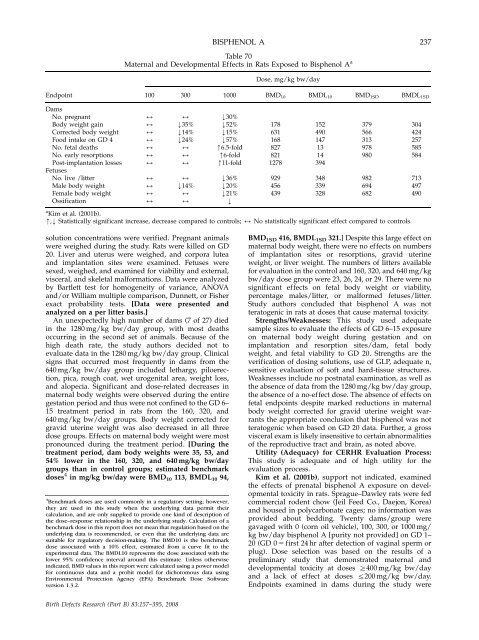
BISPHENOL A 237<br />
Table 70<br />
Maternal <strong>and</strong> Developmental Effects in Rats Exposed to Bisphenol A a<br />
Dose, mg/kg bw/day<br />
Endpoint 100 300 1000 BMD10 BMDL10 BMD1SD BMDL1SD<br />
Dams<br />
No. pregnant<br />
Body weight gain<br />
Corrected body weight<br />
Food intake <strong>on</strong> GD 4<br />
No. fetal deaths<br />
No. early resorpti<strong>on</strong>s<br />
Post-implantati<strong>on</strong> losses<br />
Fetuses<br />
No. live /litter<br />
Male body weight<br />
Female body weight<br />
Ossificati<strong>on</strong><br />
2<br />
2<br />
2<br />
2<br />
2<br />
2<br />
2<br />
2<br />
2<br />
2<br />
2<br />
2<br />
k35%<br />
k14%<br />
k24%<br />
2<br />
2<br />
2<br />
2<br />
k14%<br />
2<br />
2<br />
k30%<br />
k52%<br />
k15%<br />
k57%<br />
m6.5-fold<br />
m6-fold<br />
m11-fold<br />
k36%<br />
k20%<br />
k21%<br />
k<br />
a Kim et al. (2001b).<br />
m,k Statistically significant increase, decrease compared to c<strong>on</strong>trols; 2 No statistically significant effect compared to c<strong>on</strong>trols.<br />
soluti<strong>on</strong> c<strong>on</strong>centrati<strong>on</strong>s were verified. Pregnant animals<br />
were weighed during <strong>the</strong> study. Rats were killed <strong>on</strong> GD<br />
20. Liver <strong>and</strong> uterus were weighed, <strong>and</strong> corpora lutea<br />
<strong>and</strong> implantati<strong>on</strong> sites were examined. Fetuses were<br />
sexed, weighed, <strong>and</strong> examined for viability <strong>and</strong> external,<br />
visceral, <strong>and</strong> skeletal malformati<strong>on</strong>s. Data were analyzed<br />
by Bartlett test for homogeneity of variance, ANOVA<br />
<strong>and</strong>/or William multiple comparis<strong>on</strong>, Dunnett, or Fisher<br />
exact probability tests. [Data were presented <strong>and</strong><br />
analyzed <strong>on</strong> a per litter basis.]<br />
An unexpectedly high number of dams (7 of 27) died<br />
in <strong>the</strong> 1280 mg/kg bw/day group, with most deaths<br />
occurring in <strong>the</strong> sec<strong>on</strong>d set of animals. Because of <strong>the</strong><br />
high death rate, <strong>the</strong> study authors decided not to<br />
evaluate data in <strong>the</strong> 1280 mg/kg bw/day group. Clinical<br />
signs that occurred most frequently in dams from <strong>the</strong><br />
640 mg/kg bw/day group included lethargy, piloerecti<strong>on</strong>,<br />
pica, rough coat, wet urogenital area, weight loss,<br />
<strong>and</strong> alopecia. Significant <strong>and</strong> dose-related decreases in<br />
maternal body weights were observed during <strong>the</strong> entire<br />
gestati<strong>on</strong> period <strong>and</strong> thus were not c<strong>on</strong>fined to <strong>the</strong> GD 6–<br />
15 treatment period in rats from <strong>the</strong> 160, 320, <strong>and</strong><br />
640 mg/kg bw/day groups. Body weight corrected for<br />
gravid uterine weight was also decreased in all three<br />
dose groups. Effects <strong>on</strong> maternal body weight were most<br />
pr<strong>on</strong>ounced during <strong>the</strong> treatment period. [During <strong>the</strong><br />
treatment period, dam body weights were 35, 53, <strong>and</strong><br />
54% lower in <strong>the</strong> 160, 320, <strong>and</strong> 640 mg/kg bw/day<br />
groups than in c<strong>on</strong>trol groups; estimated benchmark<br />
doses 4 in mg/kg bw/day were BMD10 113, BMDL10 94,<br />
4 Benchmark doses are used comm<strong>on</strong>ly in a regulatory setting; however,<br />
<strong>the</strong>y are used in this study when <strong>the</strong> underlying data permit <strong>the</strong>ir<br />
calculati<strong>on</strong>, <strong>and</strong> are <strong>on</strong>ly supplied to provide <strong>on</strong>e kind of descripti<strong>on</strong> of<br />
<strong>the</strong> dose–resp<strong>on</strong>se relati<strong>on</strong>ship in <strong>the</strong> underlying study. Calculati<strong>on</strong> of a<br />
benchmark dose in this report does not mean that regulati<strong>on</strong> based <strong>on</strong> <strong>the</strong><br />
underlying data is recommended, or even that <strong>the</strong> underlying data are<br />
suitable for regulatory decisi<strong>on</strong>-making. The BMD10 is <strong>the</strong> benchmark<br />
dose associated with a 10% effect, estimated from a curve fit to <strong>the</strong><br />
experimental data. The BMDL10 represents <strong>the</strong> dose associated with <strong>the</strong><br />
lower 95% c<strong>on</strong>fidence interval around this estimate. Unless o<strong>the</strong>rwise<br />
indicated, BMD values in this report were calculated using a power model<br />
for c<strong>on</strong>tinuous data <strong>and</strong> a probit model for dichotomous data using<br />
Envir<strong>on</strong>mental Protecti<strong>on</strong> Agency (EPA) Benchmark Dose Software<br />
versi<strong>on</strong> 1.3.2.<br />
Birth Defects Research (Part B) 83:157–395, 2008<br />
178<br />
631<br />
168<br />
827<br />
821<br />
1278<br />
929<br />
456<br />
439<br />
152<br />
490<br />
147<br />
13<br />
14<br />
394<br />
348<br />
339<br />
328<br />
379<br />
566<br />
313<br />
978<br />
980<br />
982<br />
694<br />
682<br />
304<br />
424<br />
257<br />
585<br />
584<br />
713<br />
497<br />
490<br />
BMD1SD 416, BMDL1SD 321.] Despite this large effect <strong>on</strong><br />
maternal body weight, <strong>the</strong>re were no effects <strong>on</strong> numbers<br />
of implantati<strong>on</strong> sites or resorpti<strong>on</strong>s, gravid uterine<br />
weight, or liver weight. The numbers of litters available<br />
for evaluati<strong>on</strong> in <strong>the</strong> c<strong>on</strong>trol <strong>and</strong> 160, 320, <strong>and</strong> 640 mg/kg<br />
bw/day dose group were 23, 26, 24, or 29. There were no<br />
significant effects <strong>on</strong> fetal body weight or viability,<br />
percentage males/litter, or malformed fetuses/litter.<br />
Study authors c<strong>on</strong>cluded that bisphenol A was not<br />
teratogenic in rats at doses that cause maternal toxicity.<br />
Strengths/Weaknesses: This study used adequate<br />
sample sizes to evaluate <strong>the</strong> effects of GD 6–15 exposure<br />
<strong>on</strong> maternal body weight during gestati<strong>on</strong> <strong>and</strong> <strong>on</strong><br />
implantati<strong>on</strong> <strong>and</strong> resorpti<strong>on</strong> sites/dam, fetal body<br />
weight, <strong>and</strong> fetal viability to GD 20. Strengths are <strong>the</strong><br />
verificati<strong>on</strong> of dosing soluti<strong>on</strong>s, use of GLP, adequate n,<br />
sensitive evaluati<strong>on</strong> of soft <strong>and</strong> hard-tissue structures.<br />
Weaknesses include no postnatal examinati<strong>on</strong>, as well as<br />
<strong>the</strong> absence of data from <strong>the</strong> 1280 mg/kg bw/day group,<br />
<strong>the</strong> absence of a no-effect dose. The absence of effects <strong>on</strong><br />
fetal endpoints despite marked reducti<strong>on</strong>s in maternal<br />
body weight corrected for gravid uterine weight warrants<br />
<strong>the</strong> appropriate c<strong>on</strong>clusi<strong>on</strong> that bisphenol was not<br />
teratogenic when based <strong>on</strong> GD 20 data. Fur<strong>the</strong>r, a gross<br />
visceral exam is likely insensitive to certain abnormalities<br />
of <strong>the</strong> reproductive tract <strong>and</strong> brain, as noted above.<br />
Utility (Adequacy) for CERHR Evaluati<strong>on</strong> Process:<br />
This study is adequate <strong>and</strong> of high utility for <strong>the</strong><br />
evaluati<strong>on</strong> process.<br />
Kim et al. (2001b), support not indicated, examined<br />
<strong>the</strong> effects of prenatal bisphenol A exposure <strong>on</strong> developmental<br />
toxicity in rats. Sprague–Dawley rats were fed<br />
commercial rodent chow (Jeil Feed Co., Daej<strong>on</strong>, Korea)<br />
<strong>and</strong> housed in polycarb<strong>on</strong>ate cages; no informati<strong>on</strong> was<br />
provided about bedding. Twenty dams/group were<br />
gavaged with 0 (corn oil vehicle), 100, 300, or 1000 mg/<br />
kg bw/day bisphenol A [purity not provided] <strong>on</strong> GD 1–<br />
20 (GD 0 5 first 24 hr after detecti<strong>on</strong> of vaginal sperm or<br />
plug). Dose selecti<strong>on</strong> was based <strong>on</strong> <strong>the</strong> results of a<br />
preliminary study that dem<strong>on</strong>strated maternal <strong>and</strong><br />
developmental toxicity at doses Z400 mg/kg bw/day<br />
<strong>and</strong> a lack of effect at doses r200 mg/kg bw/day.<br />
Endpoints examined in dams during <strong>the</strong> study were