Yoshida - 1981 - Fundamentals of Rice Crop Science
Yoshida - 1981 - Fundamentals of Rice Crop Science
Yoshida - 1981 - Fundamentals of Rice Crop Science
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
138 FUNDAMENTALS OF RICE CROP SCIENCE<br />
Plants<br />
Plant<br />
parts<br />
concn in<br />
culture<br />
solution<br />
(ppm)<br />
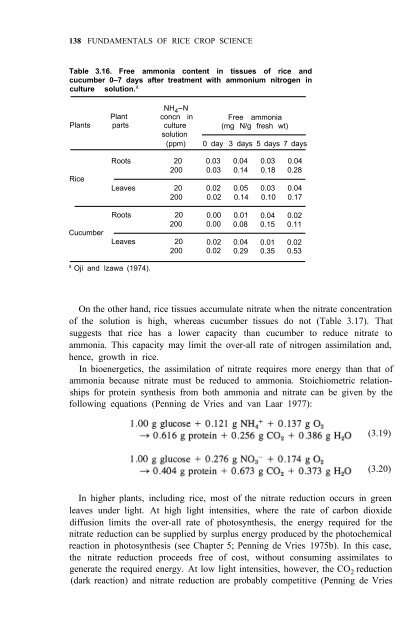
Table 3.16. Free ammonia content in tissues <strong>of</strong> rice and<br />
cucumber 0–7 days after treatment with ammonium nitrogen in<br />
culture solution. a NH 4 -N<br />
Free ammonia<br />
(mg N/g fresh wt)<br />
0 day 3 days 5 days 7 days<br />
<strong>Rice</strong><br />
Cucumber<br />
Roots 20 0.03 0.04 0.03 0.04<br />
200 0.03 0.14 0.18 0.28<br />
Leaves 20 0.02 0.05 0.03 0.04<br />
200 0.02 0.14 0.10 0.17<br />
Roots 20 0.00 0.01 0.04 0.02<br />
200 0.00 0.08 0.15 0.11<br />
Leaves 20 0.02 0.04 0.01 0.02<br />
200 0.02 0.29 0.35 0.53<br />
a Oji and lzawa (1974).<br />
On the other hand, rice tissues accumulate nitrate when the nitrate concentration<br />
<strong>of</strong> the solution is high, whereas cucumber tissues do not (Table 3.17). That<br />
suggests that rice has a lower capacity than cucumber to reduce nitrate to<br />
ammonia. This capacity may limit the over-all rate <strong>of</strong> nitrogen assimilation and,<br />
hence, growth in rice.<br />
In bioenergetics, the assimilation <strong>of</strong> nitrate requires more energy than that <strong>of</strong><br />
ammonia because nitrate must be reduced to ammonia. Stoichiometric relationships<br />
for protein synthesis from both ammonia and nitrate can be given by the<br />
following equations (Penning de Vries and van Laar 1977):<br />
(3.19)<br />
(3.20)<br />
In higher plants, including rice, most <strong>of</strong> the nitrate reduction occurs in green<br />
leaves under light. At high light intensities, where the rate <strong>of</strong> carbon dioxide<br />
diffusion limits the over-all rate <strong>of</strong> photosynthesis, the energy required for the<br />
nitrate reduction can be supplied by surplus energy produced by the photochemical<br />
reaction in photosynthesis (see Chapter 5; Penning de Vries 1975b). In this case,<br />
the nitrate reduction proceeds free <strong>of</strong> cost, without consuming assimilates to<br />
generate the required energy. At low light intensities, however, the CO 2 reduction<br />
(dark reaction) and nitrate reduction are probably competitive (Penning de Vries