Yoshida - 1981 - Fundamentals of Rice Crop Science
Yoshida - 1981 - Fundamentals of Rice Crop Science
Yoshida - 1981 - Fundamentals of Rice Crop Science
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
170 FUNDAMENTALS OF RICE CROP SCIENCE<br />
free hydrogen sulfide can really be present in concentrations toxic to rice in the soil<br />
solution <strong>of</strong> submerged soils.<br />
The first approach is to calculate H 2 S concentrations based on the chemical<br />
equilibria. As is understood from equation 3.31, the concentration <strong>of</strong> H 2S increases<br />
with a decrease in pH:<br />
p H 2 S = 2 pH – p Fe 2+ – 3.52. (3.36)<br />
Similarly, the concentration <strong>of</strong> Fe 2+ also increases with a decrease in pH:<br />
Combining these two equations:<br />
p Fe 2+ = 2 pH – 10.8. (3.37)<br />
p H 2 S = 2 pH – (2 pH – 10.8) – 3.52<br />
= 10.8 – 3.52 = 7.28. (3.38)<br />
[H 2S] = 10 –7.28 mol/liter = constant<br />
= 5 × l0 –8 mol/liter = 0.0017 ppm. (3.39)<br />
Thus, in the presence <strong>of</strong> sufficiently large amounts <strong>of</strong> ferrous iron such as in<br />
latosolic soils, the concentration <strong>of</strong> H 2 S is independent <strong>of</strong> solution pH, and that<br />
concentration is far below the critical toxic level (Ponnamperuma 1965). H 2S<br />
toxicity, however, may occur in soils low in free iron oxides, such as degraded<br />
paddy fields in Japan.<br />
Tanaka et al (1968a) considered the FeCO 3 – FeS system in the presence <strong>of</strong> the<br />
high partial pressure <strong>of</strong> carbon dioxide in submerged soils. High concentrations <strong>of</strong><br />
both ferrous iron and hydrogen sulfide can be present in the soil solution provided<br />
carbon dioxide is produced faster than hydrogen sulfide. FeS is converted to<br />
FeCO 3 by carbon dioxide and, as a consequence, free hydrogen sulfide comes into<br />
solution. Thus, two theoretical considerations lead to entirely different conclusions.<br />
The second approach is to measure hydrogen sulfide concentrations experimentally.<br />
The most common method to measure free hydrogen sulfide in the laboratory<br />
is to bubble the incubated soil with nitrogen (Suzuki and Shiga 1953, Yamane<br />
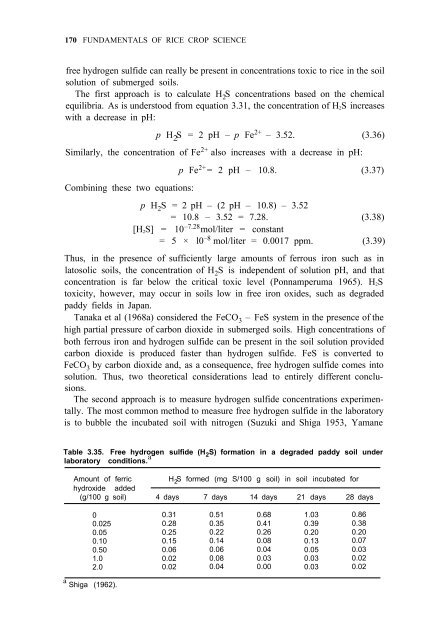
Table 3.35. Free hydrogen sulfide (H 2 S) formation in a degraded paddy soil under<br />
laboratory conditions. a<br />
Amount <strong>of</strong> ferric H 2 S formed (mg S/100 g soil) in soil incubated for<br />
hydroxide added<br />
(g/100 g soil) 4 days 7 days 14 days 21 days 28 days<br />
a Shiga (1962).<br />
0 0.31 0.51 0.68 1.03 0.86<br />
0.025 0.28 0.35 0.41 0.39 0.38<br />
0.05 0.25 0.22 0.26 0.20 0.20<br />
0.10 0.15 0.14 0.08 0.13 0.07<br />
0.50 0.06 0.06 0.04 0.05 0.03<br />
1.0 0.02 0.08 0.03 0.03 0.02<br />
2.0 0.02 0.04 0.00 0.03 0.02