A/HRC/23/51 - Office of the High Commissioner on Human Rights
A/HRC/23/51 - Office of the High Commissioner on Human Rights
A/HRC/23/51 - Office of the High Commissioner on Human Rights
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
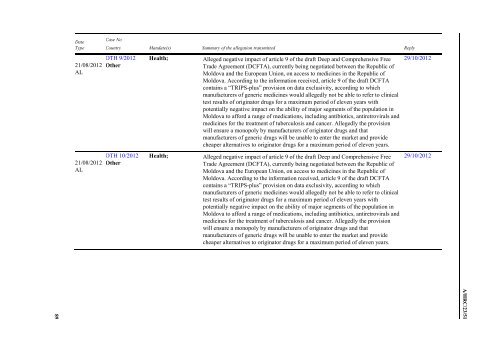
Date<br />
Type<br />
21/08/2012<br />
AL<br />
21/08/2012<br />
AL<br />
Case No<br />
Country Mandate(s) Summary <str<strong>on</strong>g>of</str<strong>on</strong>g> <str<strong>on</strong>g>the</str<strong>on</strong>g> allegati<strong>on</strong> transmitted Reply<br />
OTH 9/2012<br />
O<str<strong>on</strong>g>the</str<strong>on</strong>g>r<br />
OTH 10/2012<br />
O<str<strong>on</strong>g>the</str<strong>on</strong>g>r<br />
Health;<br />
Health;<br />
Alleged negative impact <str<strong>on</strong>g>of</str<strong>on</strong>g> article 9 <str<strong>on</strong>g>of</str<strong>on</strong>g> <str<strong>on</strong>g>the</str<strong>on</strong>g> draft Deep and Comprehensive Free<br />
Trade Agreement (DCFTA), currently being negotiated between <str<strong>on</strong>g>the</str<strong>on</strong>g> Republic <str<strong>on</strong>g>of</str<strong>on</strong>g><br />
Moldova and <str<strong>on</strong>g>the</str<strong>on</strong>g> European Uni<strong>on</strong>, <strong>on</strong> access to medicines in <str<strong>on</strong>g>the</str<strong>on</strong>g> Republic <str<strong>on</strong>g>of</str<strong>on</strong>g><br />
Moldova. According to <str<strong>on</strong>g>the</str<strong>on</strong>g> informati<strong>on</strong> received, article 9 <str<strong>on</strong>g>of</str<strong>on</strong>g> <str<strong>on</strong>g>the</str<strong>on</strong>g> draft DCFTA<br />
c<strong>on</strong>tains a ―TRIPS-plus‖ provisi<strong>on</strong> <strong>on</strong> data exclusivity, according to which<br />
manufacturers <str<strong>on</strong>g>of</str<strong>on</strong>g> generic medicines would allegedly not be able to refer to clinical<br />
test results <str<strong>on</strong>g>of</str<strong>on</strong>g> originator drugs for a maximum period <str<strong>on</strong>g>of</str<strong>on</strong>g> eleven years with<br />
potentially negative impact <strong>on</strong> <str<strong>on</strong>g>the</str<strong>on</strong>g> ability <str<strong>on</strong>g>of</str<strong>on</strong>g> major segments <str<strong>on</strong>g>of</str<strong>on</strong>g> <str<strong>on</strong>g>the</str<strong>on</strong>g> populati<strong>on</strong> in<br />
Moldova to afford a range <str<strong>on</strong>g>of</str<strong>on</strong>g> medicati<strong>on</strong>s, including antibiotics, antiretrovirals and<br />
medicines for <str<strong>on</strong>g>the</str<strong>on</strong>g> treatment <str<strong>on</strong>g>of</str<strong>on</strong>g> tuberculosis and cancer. Allegedly <str<strong>on</strong>g>the</str<strong>on</strong>g> provisi<strong>on</strong><br />
will ensure a m<strong>on</strong>opoly by manufacturers <str<strong>on</strong>g>of</str<strong>on</strong>g> originator drugs and that<br />
manufacturers <str<strong>on</strong>g>of</str<strong>on</strong>g> generic drugs will be unable to enter <str<strong>on</strong>g>the</str<strong>on</strong>g> market and provide<br />
cheaper alternatives to originator drugs for a maximum period <str<strong>on</strong>g>of</str<strong>on</strong>g> eleven years.<br />
Alleged negative impact <str<strong>on</strong>g>of</str<strong>on</strong>g> article 9 <str<strong>on</strong>g>of</str<strong>on</strong>g> <str<strong>on</strong>g>the</str<strong>on</strong>g> draft Deep and Comprehensive Free<br />
Trade Agreement (DCFTA), currently being negotiated between <str<strong>on</strong>g>the</str<strong>on</strong>g> Republic <str<strong>on</strong>g>of</str<strong>on</strong>g><br />
Moldova and <str<strong>on</strong>g>the</str<strong>on</strong>g> European Uni<strong>on</strong>, <strong>on</strong> access to medicines in <str<strong>on</strong>g>the</str<strong>on</strong>g> Republic <str<strong>on</strong>g>of</str<strong>on</strong>g><br />
Moldova. According to <str<strong>on</strong>g>the</str<strong>on</strong>g> informati<strong>on</strong> received, article 9 <str<strong>on</strong>g>of</str<strong>on</strong>g> <str<strong>on</strong>g>the</str<strong>on</strong>g> draft DCFTA<br />
c<strong>on</strong>tains a ―TRIPS-plus‖ provisi<strong>on</strong> <strong>on</strong> data exclusivity, according to which<br />
manufacturers <str<strong>on</strong>g>of</str<strong>on</strong>g> generic medicines would allegedly not be able to refer to clinical<br />
test results <str<strong>on</strong>g>of</str<strong>on</strong>g> originator drugs for a maximum period <str<strong>on</strong>g>of</str<strong>on</strong>g> eleven years with<br />
potentially negative impact <strong>on</strong> <str<strong>on</strong>g>the</str<strong>on</strong>g> ability <str<strong>on</strong>g>of</str<strong>on</strong>g> major segments <str<strong>on</strong>g>of</str<strong>on</strong>g> <str<strong>on</strong>g>the</str<strong>on</strong>g> populati<strong>on</strong> in<br />
Moldova to afford a range <str<strong>on</strong>g>of</str<strong>on</strong>g> medicati<strong>on</strong>s, including antibiotics, antiretrovirals and<br />
medicines for <str<strong>on</strong>g>the</str<strong>on</strong>g> treatment <str<strong>on</strong>g>of</str<strong>on</strong>g> tuberculosis and cancer. Allegedly <str<strong>on</strong>g>the</str<strong>on</strong>g> provisi<strong>on</strong><br />
will ensure a m<strong>on</strong>opoly by manufacturers <str<strong>on</strong>g>of</str<strong>on</strong>g> originator drugs and that<br />
manufacturers <str<strong>on</strong>g>of</str<strong>on</strong>g> generic drugs will be unable to enter <str<strong>on</strong>g>the</str<strong>on</strong>g> market and provide<br />
cheaper alternatives to originator drugs for a maximum period <str<strong>on</strong>g>of</str<strong>on</strong>g> eleven years.<br />
29/10/2012<br />
29/10/2012<br />
A/<str<strong>on</strong>g>HRC</str<strong>on</strong>g>/<str<strong>on</strong>g>23</str<strong>on</strong>g>/<str<strong>on</strong>g>51</str<strong>on</strong>g><br />
89