LVR-Klinikum Düsseldorf Hospital of the Heinrich-Heine University ...
LVR-Klinikum Düsseldorf Hospital of the Heinrich-Heine University ...
LVR-Klinikum Düsseldorf Hospital of the Heinrich-Heine University ...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
<strong>LVR</strong>-KLINIKUM DÜsseLDORF – hOsPITaL OF The heINRIch-heINe UNIVeRsITY DÜsseLDORF<br />
Serum prolactin (µIU/ml)<br />
as well as Pr<strong>of</strong>essor Hippius and Dr. Grohmann (Munich).<br />
The system is used to record clinically significant severe<br />
side effects. All recorded cases are discussed in central<br />
conferences, and <strong>the</strong> correlation between undesirable<br />
effects and <strong>the</strong> medication administered is evaluated. Biannual<br />
investigations <strong>of</strong> census samples allow a comparative<br />
estimation <strong>of</strong> <strong>the</strong> relative frequency <strong>of</strong> undesirable drug<br />
side effects for various psychotropic drugs. In addition, case<br />
documentation is used for <strong>the</strong> casuistic analysis <strong>of</strong> unusual,<br />
new side effects.<br />
104<br />
400<br />
360<br />
320<br />
280<br />
240<br />
200<br />
160<br />
800<br />
400<br />
250<br />
200<br />
150<br />
100<br />
50<br />
0<br />
-50<br />
Change in prolactin level (µIU/ml) 1.200<br />
Comparison group<br />
Patients<br />
120 -40 -20 0 20 40 60 80 100 120 140<br />
Time (min)<br />
Control group Unmedicated Medicated<br />
(n=12) (n=6) (n=14)<br />
Serum cortisol (ng/ml)<br />
200<br />
180<br />
160<br />
140<br />
120<br />
100<br />
80<br />
Comparison group<br />
Patients<br />
60 -40 -20 0 20 40 60 80 100 120 140<br />
Time (min)<br />
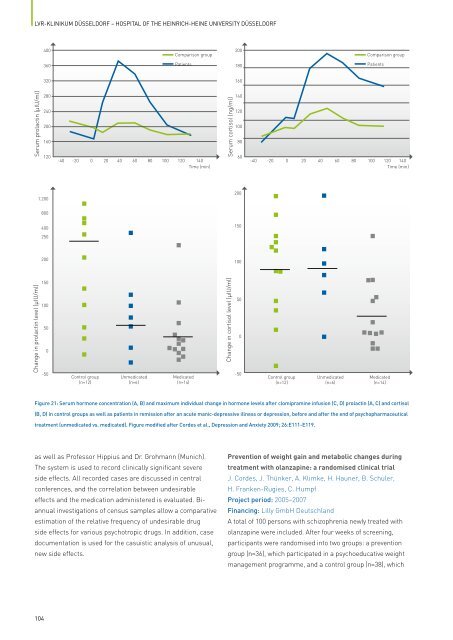
Figure 21: Serum hormone concentration (A, B) and maximum individual change in hormone levels after clomipramine infusion (C, D) prolactin (A, C) and cortisol<br />
(B, D) in control groups as well as patients in remission after an acute manic-depressive illness or depression, before and after <strong>the</strong> end <strong>of</strong> psychopharmaceutical<br />
treatment (unmedicated vs. medicated). Figure modified after Cordes et al., Depression and Anxiety 2009; 26:E111-E119.<br />
Change in cortisol level (µIU/ml)<br />
200<br />
150<br />
100<br />
50<br />
0<br />
-50<br />
Control group Unmedicated Medicated<br />
(n=12) (n=6) (n=14)<br />
Prevention <strong>of</strong> weight gain and metabolic changes during<br />
treatment with olanzapine: a randomised clinical trial<br />
J. Cordes, J. Thünker, A. Klimke, H. Hauner, B. Schuler,<br />
H. Franken-Rugies, C. Humpf<br />
Project period: 2005–2007<br />
Financing: Lilly GmbH Deutschland<br />
A total <strong>of</strong> 100 persons with schizophrenia newly treated with<br />
olanzapine were included. After four weeks <strong>of</strong> screening,<br />
participants were randomised into two groups: a prevention<br />
group (n=36), which participated in a psychoeducative weight<br />
management programme, and a control group (n=38), which