LVR-Klinikum Düsseldorf Hospital of the Heinrich-Heine University ...
LVR-Klinikum Düsseldorf Hospital of the Heinrich-Heine University ...
LVR-Klinikum Düsseldorf Hospital of the Heinrich-Heine University ...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
<strong>LVR</strong>-KLINIKUM DÜsseLDORF – hOsPITaL OF The heINRIch-heINe UNIVeRsITY DÜsseLDORF<br />
Alpha<br />
evaluation is being used to address <strong>the</strong> question <strong>of</strong> whe<strong>the</strong>r<br />
EEG alterations in MCI or beginning AD are associated with<br />
<strong>the</strong> course <strong>of</strong> <strong>the</strong> illness and drug effects (v. Figures 17 and<br />
18).<br />
Study E 2.2: Pharmacological treatment <strong>of</strong><br />
patients with mild cognitive impairment<br />
The subject <strong>of</strong> this 24-month treatment study within<br />
<strong>the</strong> context <strong>of</strong> <strong>the</strong> Competence Network on Dementia<br />
was <strong>the</strong> preventive effectiveness <strong>of</strong> early anti-dementia<br />
treatment in patients with mild cognitive impairment (MCI).<br />
In <strong>Düsseldorf</strong>, 30 patients were included in <strong>the</strong> study.<br />
They were treated in a double-blind study design with<br />
placebo, <strong>the</strong> acetylcholinesterase inhibitor galantamine or<br />
with a combination <strong>of</strong> galantamine and memantine. The<br />
administration <strong>of</strong> study drugs in this study was terminated<br />
prematurely because two major, international, multicentre<br />
98<br />
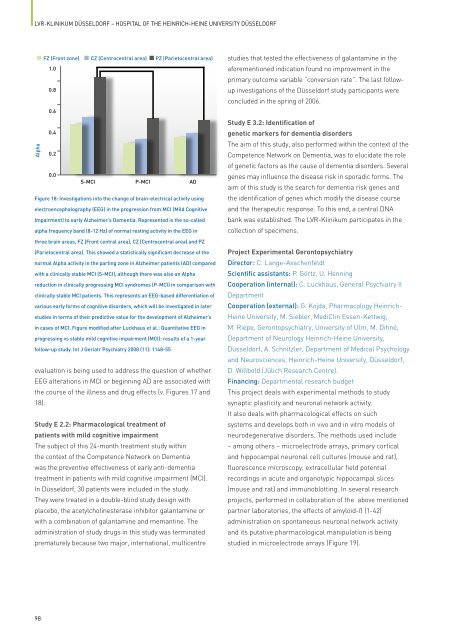
FZ (Front zone) CZ (Centrocentral area) PZ (Parietocentral area)<br />
1.0<br />
0.8<br />
0.6<br />
0.4<br />
0.2<br />
0.0<br />
S-MCI P-MCI AD<br />
Figure 18: Investigations into <strong>the</strong> change <strong>of</strong> brain-electrical activity using<br />
electroencephalography (EEG) in <strong>the</strong> progression from MCI (Mild Cognitive<br />
Impairment) to early Alzheimer’s Dementia. Represented is <strong>the</strong> so-called<br />
alpha frequency band (8-12 Hz) <strong>of</strong> normal resting activity in <strong>the</strong> EEG in<br />
three brain areas, FZ (Front central area), CZ (Centrocentral area) and PZ<br />
(Parietocentral area). This showed a statistically significant decrease <strong>of</strong> <strong>the</strong><br />
normal Alpha activity in <strong>the</strong> parting zone in Alzheimer patients (AD) compared<br />
with a clinically stable MCI (S-MCI), although <strong>the</strong>re was also an Alpha<br />
reduction in clinically progressing MCI syndromes (P-MCI) in comparison with<br />
clinically stable MCI patients. This represents an EEG-based differentiation <strong>of</strong><br />
various early forms <strong>of</strong> cognitive disorders, which will be investigated in later<br />
studies in terms <strong>of</strong> <strong>the</strong>ir predictive value for <strong>the</strong> development <strong>of</strong> Alzheimer’s<br />
in cases <strong>of</strong> MCI. Figure modified after Luckhaus et al.: Quantitative EEG in<br />
progressing vs stable mild cognitive impairment (MCI): results <strong>of</strong> a 1-year<br />
follow-up study. Int J Geriatr Psychiatry 2008 (11): 1148-55<br />
studies that tested <strong>the</strong> effectiveness <strong>of</strong> galantamine in <strong>the</strong><br />
aforementioned indication found no improvement in <strong>the</strong><br />
primary outcome variable “conversion rate”. The last followup<br />
investigations <strong>of</strong> <strong>the</strong> <strong>Düsseldorf</strong> study participants were<br />
concluded in <strong>the</strong> spring <strong>of</strong> 2006.<br />
Study E 3.2: Identification <strong>of</strong><br />
genetic markers for dementia disorders<br />
The aim <strong>of</strong> this study, also performed within <strong>the</strong> context <strong>of</strong> <strong>the</strong><br />
Competence Network on Dementia, was to elucidate <strong>the</strong> role<br />
<strong>of</strong> genetic factors as <strong>the</strong> cause <strong>of</strong> dementia disorders. Several<br />
genes may influence <strong>the</strong> disease risk in sporadic forms. The<br />
aim <strong>of</strong> this study is <strong>the</strong> search for dementia risk genes and<br />
<strong>the</strong> identification <strong>of</strong> genes which modify <strong>the</strong> disease course<br />
and <strong>the</strong> <strong>the</strong>rapeutic response. To this end, a central DNA<br />
bank was established. The <strong>LVR</strong>-<strong>Klinikum</strong> participates in <strong>the</strong><br />
collection <strong>of</strong> specimens.<br />
Project Experimental Gerontopsychiatry<br />
Director: C. Lange-Asschenfeldt<br />
Scientific assistants: P. Görtz, U. Henning<br />
Cooperation (internal): C. Luckhaus, General Psychiatry II<br />
Department<br />
Cooperation (external): G. Kojda, Pharmacology <strong>Heinrich</strong>-<br />
<strong>Heine</strong> <strong>University</strong>, M. Siebler, MediClin Essen-Kettwig,<br />
M. Riepe, Gerontopsychiatry, <strong>University</strong> <strong>of</strong> Ulm, M. Dihné,<br />
Department <strong>of</strong> Neurology <strong>Heinrich</strong>-<strong>Heine</strong> <strong>University</strong>,<br />
<strong>Düsseldorf</strong>, A. Schnitzler, Department <strong>of</strong> Medical Psychology<br />
and Neurosciences, <strong>Heinrich</strong>-<strong>Heine</strong> <strong>University</strong>, <strong>Düsseldorf</strong>,<br />
D. Willbold (Jülich Research Centre).<br />
Financing: Departmental research budget<br />
This project deals with experimental methods to study<br />
synaptic plasticity and neuronal network activity.<br />
It also deals with pharmacological effects on such<br />
systems and develops both in vivo and in vitro models <strong>of</strong><br />
neurodegenerative disorders. The methods used include<br />
– among o<strong>the</strong>rs – microelectrode arrays, primary cortical<br />
and hippocampal neuronal cell cultures (mouse and rat),<br />
fluorescence microscopy, extracellular field potential<br />
recordings in acute and organotypic hippocampal slices<br />
(mouse and rat) and immunoblotting. In several research<br />
projects, performed in collaboration <strong>of</strong> <strong>the</strong> above mentioned<br />
partner laboratories, <strong>the</strong> effects <strong>of</strong> amyloid-ß (1-42)<br />
administration on spontaneous neuronal network activity<br />
and its putative pharmacological manipulation is being<br />
studied in microelectrode arrays (Figure 19).