LVR-Klinikum Düsseldorf Hospital of the Heinrich-Heine University ...
LVR-Klinikum Düsseldorf Hospital of the Heinrich-Heine University ...
LVR-Klinikum Düsseldorf Hospital of the Heinrich-Heine University ...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
s Archiving study documents<br />
s Supporting collaboration with CCS (see below, noncommercial<br />
studies)<br />
In non-commercial studies, <strong>the</strong> cost-effective close<br />
collaboration with <strong>the</strong> Coordination Centre for Clinical<br />
Studies (CCS; Director: C. Ohmann) <strong>of</strong> <strong>the</strong> <strong>Heinrich</strong>-<strong>Heine</strong><br />
<strong>University</strong> is obligatory in most cases. Collaboration is based<br />
on a standard quality assurance with joint SOPs. The SOPs,<br />
available in <strong>the</strong> CCS, are made available by <strong>the</strong> Study Service<br />
Centre for this purpose.<br />
Support <strong>of</strong> pharmacological studies<br />
Since <strong>the</strong> foundation <strong>of</strong> <strong>the</strong> Study Service Centre in 2008, <strong>the</strong><br />
following pharmacological studies have been supported:<br />
s The effectiveness <strong>of</strong> <strong>the</strong> ENLA weight management<br />
programme in <strong>the</strong> prevention <strong>of</strong> weight gain in<br />
patients with schizophrenia treated with olanzapine:<br />
a randomised multi-centre controlled open study<br />
(ENLA = Exercise Nutrition Learning Accepting) (ENLA<br />
study)<br />
s Efficacy and Safety <strong>of</strong> Vivitrol® in Adults Completing<br />
Inpatient Treatment for Alcohol Dependence (VIVITROL<br />
study)<br />
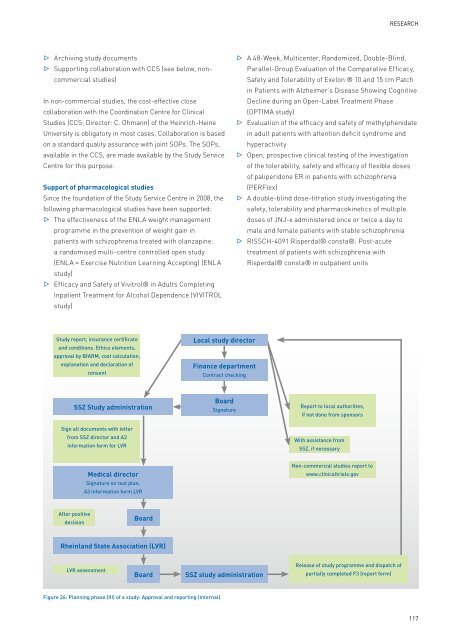
Study report, insurance certificate<br />
and conditions. Ethics elements,<br />
approval by BfARM, cost calculation,<br />
explanation and declaration <strong>of</strong><br />
consent<br />
SSZ Study administration<br />
Sign all documents with letter<br />
from SSZ director and A3<br />
information form for <strong>LVR</strong><br />
After positive<br />
decision<br />
Medical director<br />
Signature on test plan,<br />
A3 information form <strong>LVR</strong><br />
Board<br />
Rheinland State Association (<strong>LVR</strong>)<br />
<strong>LVR</strong> assessment<br />
Board<br />
Figure 26: Planning phase (III) <strong>of</strong> a study: Approval and reporting (internal)<br />
Local study director<br />
Finance department<br />
Contract checking<br />
Board<br />
Signature<br />
SSZ study administration<br />
ReseaRch<br />
s A 48-Week, Multicenter, Randomized, Double-Blind,<br />
Parallel-Group Evaluation <strong>of</strong> <strong>the</strong> Comparative Efficacy,<br />
Safety and Tolerability <strong>of</strong> Exelon ® 10 and 15 cm Patch<br />
in Patients with Alzheimer’s Disease Showing Cognitive<br />
Decline during an Open-Label Treatment Phase<br />
(OPTIMA study)<br />
s Evaluation <strong>of</strong> <strong>the</strong> efficacy and safety <strong>of</strong> methylphenidate<br />
in adult patients with attention deficit syndrome and<br />
hyperactivity<br />
s Open, prospective clinical testing <strong>of</strong> <strong>the</strong> investigation<br />
<strong>of</strong> <strong>the</strong> tolerability, safety and efficacy <strong>of</strong> flexible doses<br />
<strong>of</strong> paliperidone ER in patients with schizophrenia<br />
(PERFlex)<br />
s A double-blind dose-titration study investigating <strong>the</strong><br />
safety, tolerability and pharmacokinetics <strong>of</strong> multiple<br />
doses <strong>of</strong> JNJ-x administered once or twice a day to<br />
male and female patients with stable schizophrenia<br />
s RISSCH-4091 Risperdal® consta®: Post-acute<br />
treatment <strong>of</strong> patients with schizophrenia with<br />
Risperdal® consta® in outpatient units<br />
Report to local authorities,<br />
if not done from sponsors<br />
With assistance from<br />
SSZ, if necessary<br />
Non-commercial studies report to<br />
www.clinicaltrials.gov<br />
Release <strong>of</strong> study programme and dispatch <strong>of</strong><br />
partially completed F3 (report form)<br />
117