Solar Energy Perspectives - IEA
Solar Energy Perspectives - IEA
Solar Energy Perspectives - IEA
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
<strong>Solar</strong> <strong>Energy</strong> <strong>Perspectives</strong>: <strong>Solar</strong> fuels<br />
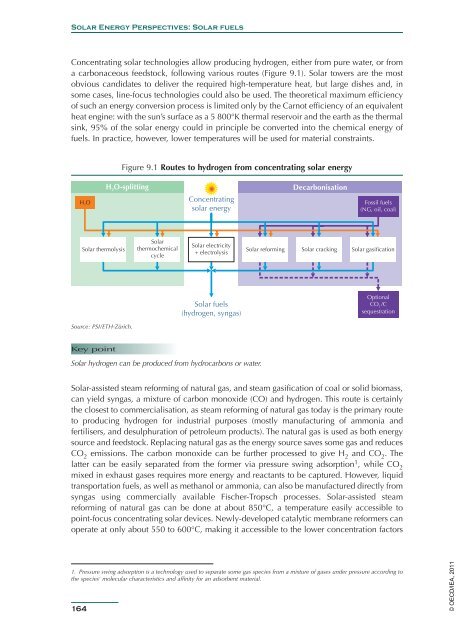
Concentrating solar technologies allow producing hydrogen, either from pure water, or from<br />
a carbonaceous feedstock, following various routes (Figure 9.1). <strong>Solar</strong> towers are the most<br />
obvious candidates to deliver the required high-temperature heat, but large dishes and, in<br />
some cases, line-focus technologies could also be used. The theoretical maximum efficiency<br />
of such an energy conversion process is limited only by the Carnot efficiency of an equivalent<br />
heat engine: with the sun’s surface as a 5 800°K thermal reservoir and the earth as the thermal<br />
sink, 95% of the solar energy could in principle be converted into the chemical energy of<br />
fuels. In practice, however, lower temperatures will be used for material constraints.<br />
Figure 9.1 Routes to hydrogen from concentrating solar energy<br />
H2O-splitting<br />
Decarbonisation<br />
H 2 O<br />
Concentrating<br />
Fossil fuels<br />
solar energy<br />
(NG, oil, coal)<br />
<strong>Solar</strong> thermolysis<br />
<strong>Solar</strong><br />
thermochemical<br />
cycle<br />
<strong>Solar</strong> electricity<br />
+ electrolysis<br />
<strong>Solar</strong> reforming <strong>Solar</strong> cracking <strong>Solar</strong> gasification<br />
<strong>Solar</strong> fuels<br />
(hydrogen, syngas)<br />
Optional<br />
CO 2 /C<br />
sequestration<br />
Source: PSI/ETH-Zürich.<br />
Key point<br />
<strong>Solar</strong> hydrogen can be produced from hydrocarbons or water.<br />
<strong>Solar</strong>-assisted steam reforming of natural gas, and steam gasification of coal or solid biomass,<br />
can yield syngas, a mixture of carbon monoxide (CO) and hydrogen. This route is certainly<br />
the closest to commercialisation, as steam reforming of natural gas today is the primary route<br />
to producing hydrogen for industrial purposes (mostly manufacturing of ammonia and<br />
fertilisers, and desulphuration of petroleum products). The natural gas is used as both energy<br />
source and feedstock. Replacing natural gas as the energy source saves some gas and reduces<br />
CO 2 emissions. The carbon monoxide can be further processed to give H 2 and CO 2 . The<br />
latter can be easily separated from the former via pressure swing adsorption 1 , while CO 2<br />
mixed in exhaust gases requires more energy and reactants to be captured. However, liquid<br />
transportation fuels, as well as methanol or ammonia, can also be manufactured directly from<br />
syngas using commercially available Fischer-Tropsch processes. <strong>Solar</strong>-assisted steam<br />
reforming of natural gas can be done at about 850°C, a temperature easily accessible to<br />
point-focus concentrating solar devices. Newly-developed catalytic membrane reformers can<br />
operate at only about 550 to 600°C, making it accessible to the lower concentration factors<br />
1. Pressure swing adsorption is a technology used to separate some gas species from a mixture of gases under pressure according to<br />
the species' molecular characteristics and affinity for an adsorbent material.<br />
164<br />
© OECD/<strong>IEA</strong>, 2011