epr-method (2003) - IAEA Publications - International Atomic Energy ...
epr-method (2003) - IAEA Publications - International Atomic Energy ...
epr-method (2003) - IAEA Publications - International Atomic Energy ...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
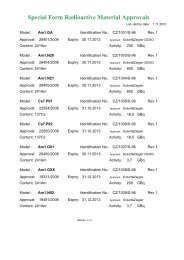
Table A8-I D Values [TBq]<br />
Sources and material 105<br />
Radionuclide<br />
106<br />
D 1<br />
107<br />
D 2<br />
H-3 UL 108 2.E+03 109<br />
C-14 2.E+05 5.E+01<br />
P-32 1.E+01 2.E+01<br />
S-35 4.E+04 6.E+01<br />
Cl-36 3.E+02 2.E+01 110<br />
Cr-51 2.E+00 5.E+03<br />
Fe-55 UL 8.E+02<br />
Co-57 7.E-01 4.E+02<br />
Co-60 3.E-02 3.E+01<br />
Ni-63 UL 6.E+01<br />
Zn-65 1.E-01 3.E+02<br />
Ge-68 7.E-02 2.E+01<br />
Se-75 2.E-01 2.E+02<br />
Kr-85 3.E+01 2.E+03 111<br />
Sr-89 2.E+01 2.E+01<br />
Sr-90 (Y-90) 112 4.E+00 1.E+00<br />
Y-90 5.E+00 1.E+01 113<br />
Y-91 8.E+00 2.E+01<br />
Zr-95<br />
(Nb-95m/Nb-95) 112 4.E-02 1.E+01<br />
Nb-95 9.E-02 6.E+01<br />
Mo-99 (Tc-99m) 112 3.E-01 2.E+01 113<br />
Tc-99m 7.E-01 7.E+02 113<br />
Ru-103 (Rh-103m) 112 1.E-01 3.E+01<br />
105 The amount of material if not controlled in the public domain (i.e. allowing removal of shielding or allowing dispersal)<br />
that could give rise to exposure resulting in a permanent injury that would decrease the quality of life. Appendix 18 provides<br />
a plain language statement of the risk to the public for an uncontrolled dangerous source.<br />
106 This is for external exposure and applies to both dispersible and non-dispersible materials. It is the amount of material<br />
without shielding that, if carried in a pocket for 10 hours could result in a severe injury (deliver 25 Gy at 2.0 cm in 10 hours)<br />
except where the amount needed would be too big to put in a pocket for which it is the amount that could be life threatening<br />
if people are near it for a long time (days–weeks) (deliver 0.01 Gy/h at 1 m). Both gamma emissions and bremsstrahlung<br />
from beta and conversion electrons were considered. They were based, except for neutron sources, on absorbed dose<br />
coefficients from Ref. [48].<br />
107 This is the quantity of material that if dispersed could deliver long term doses that could result in permanent injuries that<br />
decrease the quality of life. Airborne dispersal by fire or explosion, inadvertent ingestion and intentional contamination of<br />
water was considered. For low LET emitters, quantities that can result in 6 Gy to the lung, 1 Gy to the red bone marrow, or 5<br />
Gy to the thyroid delivered over two days [2, 3, 47] following intake and for high LET emitters (e.g. alpha) quantities that<br />
can deliver 25 Gy to the lung over one year [46] were considered to deliver long term doses that are at the threshold for the<br />
onset of permanent injuries that would decrease the quality of life. The absorbed doses from intake were, except for Cf-252,<br />
based on absorbed dose coefficients from Ref. [48].<br />
108 UL - Unlimited quantity - Emergency planning for dealing with radiological consequences is not recommended.<br />
109 Assumes skin absorption doubles the absorbed dose from intake via inhalation.<br />
110 Emergencies involving these amounts of these radionuclides may result in airborne concentrations exceeding the<br />
immediate danger to life or health (IDLH) [35] concentration for chemical toxicity. Emergency arrangements to deal with the<br />
chemical toxicity and perceived risks may be warranted.<br />
111 The amount of Kr-85 that can deliver 1 Gy from submersion in 0.5 hours if 100% is released into a 300m 3 room – the<br />
exposure scenario from Ref. [42].<br />
112 It was assumed that this source is up to 10 years old at the time of the emergency and that the D value is the quantity of the<br />
parent remaining at the time of the emergency. The D values were calculated considering both the parent and important decay<br />
products that are present after up to 10 years (radionuclide shown in parenthesis). Decay products with a half-life of less than<br />
1 year can be assumed to be in equilibrium with their parents.<br />
113 Not a long term concern as it is short lived (has a half life of less than about 7 days) and within one month (and in most<br />
cases much less), the radiological hazard will be greatly diminished.<br />
182