libro de ciencias naturales noveno grado jrd2013
libro de ciencias naturales noveno grado jrd2013
libro de ciencias naturales noveno grado jrd2013
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
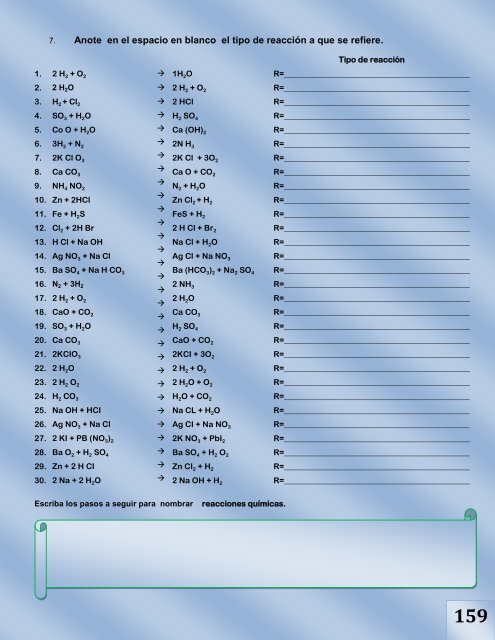
7. Anote en el espacio en blanco el tipo <strong>de</strong> reacción a que se refiere.<br />
Tipo <strong>de</strong> reacción<br />
1. 2 H 2 + O 2 1H 2O R=______________________________________________<br />
2. 2 H2O 2 H 2 + O 2 R=______________________________________________<br />
3.<br />
4.<br />
5.<br />
6.<br />
7.<br />
8.<br />
9.<br />
10.<br />
11.<br />
12.<br />
13.<br />
14.<br />
15.<br />
16.<br />
17.<br />
18.<br />
19.<br />
20.<br />
21.<br />
22.<br />
23.<br />
24.<br />
25.<br />
26.<br />
27.<br />
28.<br />
29.<br />
30.<br />
H 2 + Cl 2<br />
SO 3 + H 2O<br />
Co O + H 2O<br />
3H 2 + N 2<br />
2K Cl O 3<br />
Ca CO 3<br />
NH 4 NO 2<br />
Zn + 2HCl<br />
Fe + H 2S<br />
Cl 2 + 2H Br<br />
H Cl + Na OH<br />
Ag NO 3 + Na Cl<br />
Ba SO 4 + Na H CO 3<br />
N2 + 3H2<br />
2 H 2 + O 2<br />
CaO + CO 2<br />
SO 3 + H 2O<br />
Ca CO 3<br />
2KCIO 3<br />
2 H 2O<br />
2 H 2 O 2<br />
H 2 CO 3<br />
Na OH + HCI<br />
Ag NO 3 + Na CI<br />
2 KI + PB (NO 3) 2<br />
Ba O 2 + H 2 SO 4<br />
Zn + 2 H CI<br />
2 Na + 2 H 2O<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
2 HCl<br />
H 2 SO 4<br />
Ca (OH) 2<br />
2N H 3<br />
2K Cl + 3O 2<br />
Ca O + CO 2<br />
N 2 + H 2O<br />
Zn Cl 2 + H 2<br />
FeS + H 2<br />
2 H Cl + Br 2<br />
Na Cl + H 2O<br />
Ag Cl + Na NO 3<br />
Ba (HCO 3) 2 + Na 2 SO 4<br />
2 NH 3<br />
2 H 2O<br />
Ca CO 3<br />
H 2 SO 4<br />
CaO + CO 2<br />
2KCI + 3O 2<br />
2 H 2 + O 2<br />
2 H 2O + O 2<br />
H 2O + CO 2<br />
Na CL + H 2O<br />
Ag CI + Na NO 3<br />
2K NO 3 + PbI 2<br />
Ba SO 4 + H 2 O 2<br />
Zn CI 2 + H 2<br />
2 Na OH + H 2<br />
Escriba los pasos a seguir para nombrar reacciones químicas.<br />
R=______________________________________________<br />
R=______________________________________________<br />
R=______________________________________________<br />
R=______________________________________________<br />
R=______________________________________________<br />
R=______________________________________________<br />
R=______________________________________________<br />
R=______________________________________________<br />
R=______________________________________________<br />
R=______________________________________________<br />
R=______________________________________________<br />
R=______________________________________________<br />
R=______________________________________________<br />
R=______________________________________________<br />
R=______________________________________________<br />
R=______________________________________________<br />
R=______________________________________________<br />
R=______________________________________________<br />
R=______________________________________________<br />
R=______________________________________________<br />
R=______________________________________________<br />
R=______________________________________________<br />
R=______________________________________________<br />
R=______________________________________________<br />
R=______________________________________________<br />
R=______________________________________________<br />
R=______________________________________________<br />
R=______________________________________________<br />
159