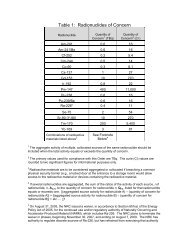
- Page 1 and 2:
NUREG-1537, Part 2 Guidelines for P
- Page 3 and 4:
AVAILABILlTY NOTICE Availability of
- Page 5 and 6:
NUREG-1537, Part 2, has been reprod
- Page 7 and 8:
Page 4 REACTOR DESCRIPTION ........
- Page 9 and 10:
- | CON1, Page 12.6 Records .......
- Page 11 and 12:
ABBOEVW7!NS IAEA International Atom
- Page 13 and 14:
INTRODUCTION Background Ts document
- Page 15 and 16:
NTRODUCMON accidents, since the maj
- Page 17 and 18:
INTRODUCnION reviewers dealing with
- Page 19 and 20:
IRODUCriON accident consequences. T
- Page 21 and 22:
1 THE FACILITY Chapter 1 of the saf
- Page 23 and 24:
* safety criteria proposed by the a
- Page 25 and 26:
THE FACILITY SAR. The technical spe
- Page 27 and 28:
Evaluaton Findings NRC does not wri
- Page 29 and 30:
THE FACILTIY Applicants are expecte
- Page 31 and 32:
* assumed inventory of fission prod
- Page 33 and 34:
ThE ForAaXy agreement with the Depa
- Page 35 and 36:
Appendix 1.1 Introduction from NURE
- Page 37 and 38:
Appendix 1.2 DOE Letter to NRC Conc
- Page 39 and 40:
2 SIT CHARACTERISCS This chapter pr
- Page 41 and 42:
SnE CHARAcrmusncs * The demographic
- Page 43 and 44:
SIE CHARAcXERIcs * historical seaso
- Page 45 and 46:
SrYI CHARAcrIsnTc other water-induc
- Page 47 and 48:
SrnE CHARAcYmusna * The geologic fe
- Page 49 and 50:
3 DESIGN OF STRUCTURES, SYSTEMS, AN
- Page 51 and 52:
DESIGN OF STRUCTURES, SYSTEM, AND C
- Page 53 and 54:
DESIGN OF SRUCrURES Sysms. AND COMP
- Page 55 and 56:
Evasion Findings - DESIGN OF STRucn
- Page 57 and 58:
DESIGN OF STRnUtURES, SYSTEMS, AND
- Page 59 and 60:
CHAPTER 4 reactor core. Subsequent
- Page 61 and 62:
CHAPTER 4 * The discussion of the f
- Page 63 and 64:
CHAPTER 4 * The control rod design
- Page 65 and 66:
CHAPTER4 * materials * compatibilit
- Page 67 and 68:
CHAPrER4 when starting the reactor
- Page 69 and 70:
CHAPT 4 * The applicant has justifi
- Page 71 and 72:
CHAFtE4 characteristics of the reac
- Page 73 and 74:
CHAPTER4 Review Procedures The revi
- Page 75 and 76:
CHAPrER4 calculational models and a
- Page 77 and 78:
CHAPTER 4 Anticipated rearrangement
- Page 79 and 80:
CHAPTER 4 4.5.2 Reactor Core Physic
- Page 81 and 82:
CHAPTER 4 included them in the tech
- Page 83 and 84:
CHAPTER. 4 Evaluation Findings This
- Page 85 and 86:
CHAPTER 4 For a pulsing reactor, li
- Page 87 and 88:
CHAPTER5 This chapter gives the rev
- Page 89 and 90:
CHAPTER S The descriptions and disc
- Page 91 and 92:
CHAPTRS acceptable radiological dos
- Page 93 and 94:
CHAPTER 5 dissipation is accomplish
- Page 95 and 96:
CHAPTER 5 5.4 Primary Coolant Clean
- Page 97 and 98:
CHAPTERS achieved. The design ensur
- Page 99 and 100:
CHAPTERS Ewauation Findings This se
- Page 101 and 102:
CHAPTR5 Evaluation Findings This se
- Page 103 and 104:
CHAPTER5 analyzed. The reviewer sho
- Page 105 and 106:
CHAPTER 6 The accident analyses by
- Page 107 and 108:
CHAmR 6 6.1 Summary Description In
- Page 109 and 110:
CtwxR 6 Acceptance CnItenad The acc
- Page 111 and 112:
I CUAPTER6 Higher power non-power r
- Page 113 and 114:
CHAPT6 * The design that should red
- Page 115 and 116:
CHArr't 6 * The ECCS should not int
- Page 117 and 118:
7 INSTRUMENTATION ANDCONTROL SYSTEM
- Page 119 and 120:
INSTRumENTAIroN AND CoNIROL SYmtm s
- Page 121 and 122:
INSRUMENTAnON AND CONTROL SYSTEMS *
- Page 123 and 124:
_ INsTRumE3nArroN AND CONTROL SYSTE
- Page 125 and 126:
INSIIuMETrATnON MD CoNmoL SYSTEm *
- Page 127 and 128:
Acceptance Cnteria INSFRUMENTAMON A
- Page 129 and 130:
Review Procedures INTUMENTATION AND
- Page 131 and 132:
- -- IxStRUMwnTAToN AND CONTRoL Sys
- Page 133 and 134:
* . personnel and equipment protect
- Page 135 and 136:
-.... - . INSTRUMENTATION AND CONTR
- Page 137 and 138:
INSRUMENATION AD CONIMOL SYSTMS * c
- Page 139 and 140:
INSgtmrNTA1ION AND CoNmoL SySIEmm A
- Page 141 and 142:
CHAPrER 8 * The system should be de
- Page 143 and 144:
CHAPTER8 Acceptance Criteria ) The
- Page 145 and 146:
9 AUXILIARY SYSTEMS This chapter co
- Page 147 and 148:
AUXILIARY SYSEms * 0 CFR Part 20 an
- Page 149 and 150:
. AuxiLARY SYSTEMS * Methods for in
- Page 151 and 152:
Evaluation Findings AuxnARY SYSTEMS
- Page 153 and 154:
Rewew Procedures AUXILIARY SYSTEMS
- Page 155 and 156:
AuxLiARY SYSTEMS * The facility com
- Page 157 and 158:
Revew Procedures AUXLARY SYSmEm The
- Page 159 and 160:
AuxILIARY SYSTEM * analyses of the
- Page 161 and 162:
AuNARY SYSTEMS * No function or mal
- Page 163 and 164:
CHAPTER 10 accidents including malf
- Page 165 and 166:
CHAPrER 10 Areas of review should i
- Page 167 and 168:
CHAPTER 10 dimensions, materials, a
- Page 169 and 170:
CHAPt 10 integrity during all antic
- Page 171 and 172:
I CHAPTER 10 The applicant should l
- Page 173 and 174:
11 RADIATION PROTECTION-PROGRAM AND
- Page 175 and 176:
RADIAnON PRoTEcnoN PROGRAM AND WAST
- Page 177 and 178:
RADiAnoN PROTECTON PROGRAM AND WAST
- Page 179 and 180:
Acceptance Criteria RADIATIoN PRtow
- Page 181 and 182:
RADIATION PROTECION PROGRAM AND WAS
- Page 183 and 184:
RADIAnON PRoTECTION PROM AND WASTE
- Page 185 and 186:
RADAION PROTECTnON PRoORAmAND WAsTE
- Page 187 and 188:
RADIATION PRtowCioN PRoGRAm AND WAS
- Page 189 and 190:
* radiation monitors, alarms and sa
- Page 191 and 192:
RADIATnON PROTECrON PRORAM AND WAsT
- Page 193 and 194:
. RADiuTION PROTEcoN PROGRAM AND WA
- Page 195 and 196:
I - - RADiA11ON PRCoECnON PROCRAM A
- Page 197 and 198:
Revew Procedures RADIATINo PRoTEoN
- Page 199 and 200:
RADiiAoNPRaorcnoN PROGRAM AND WASTE
- Page 201 and 202: RADLATnON PRoTCTON PROGRAM AND WAST
- Page 203 and 204: CHAPTER 12 day-to-day operation of
- Page 205 and 206: CHA'rER 12 12.2 Review and Audit Ac
- Page 207 and 208: CHAPTER 12 staff has determined tha
- Page 209 and 210: CHAPE 12 to correct and prevent rec
- Page 211 and 212: CHArER 12 12.6 Records Areas of Rev
- Page 213 and 214: CHAPrER 12 * requalification docume
- Page 215 and 216: CHATRi 12 operator skills and knowl
- Page 217 and 218: CHAPTER 12 applicant's safety analy
- Page 219 and 220: Appendix 12.1 Environmental Conside
- Page 221 and 222: APPENDvc 12.1 Chemical and sanitary
- Page 223 and 224: APPENDix 12.1 Conclusion The staff
- Page 225 and 226: CHAPTER 13 The information in this
- Page 227 and 228: CHAPSER 13 * The single malfunction
- Page 229 and 230: CHITER 13 (c) (For high-powered rea
- Page 231 and 232: CHAPTER 13 or * protracted malfunct
- Page 233 and 234: CHAPTER 13 * With natural-convectio
- Page 235 and 236: CHAPTER 13 (If the MHA is not afuel
- Page 237 and 238: CHAPTDl 13 Bibliography Non-Power R
- Page 239 and 240: CHAPShR 13 U.S. Nuclear Regulatory
- Page 241 and 242: 14 TECHNCAL SPECIFICATIONS This cha
- Page 243 and 244: TEcHnICAL SpEcmcAnoms * The staff h
- Page 245 and 246: CHAPTER 15 * Areas of review should
- Page 247 and 248: CHAnM R1S * The applicant should es
- Page 249 and 250: CHAP~i~tIS Environmental Review Pro
- Page 251: CHAPD 16 system; engineered safety
- Page 255 and 256: CHAPS 16 treatment. The door or ent
- Page 257 and 258: CHAEW 16 * Requirements for reporti
- Page 259 and 260: 17 DECOMMISSIONING AN) POSSESSION-O
- Page 261 and 262: - DEcOM iSNNG AND PossEssION-ONLY A
- Page 263 and 264: DEcOmm oNG ANDPossEsoN-ONLYAmm .ES
- Page 265 and 266: Review Procedires DEamRsoNEA PosHmo
- Page 267 and 268: DEcOmmXsoNU4G AD PossEssioN-ONLY AM
- Page 269 and 270: DEcoMMiSsIoNING AND PossEssioN-ONLY
- Page 271 and 272: NRC Review of Decommissioning Plan
- Page 273 and 274: 2.2.2 Current Radiological Status o
- Page 275 and 276: DEMMM=ONMPJANS 2.4 Decommissioning
- Page 277 and 278: DECCMMISSIONI0PLANS The DP should s
- Page 279 and 280: DEco wIssioNDJGPLA controls to comp
- Page 281 and 282: - - ~DECOM SSOKMGPLANS facility. Su
- Page 283 and 284: 8 ENVIRONMENTAL REPORT ..DECOMOM=ON
- Page 285 and 286: 18 HIGH1LY ENRICHED TO LOW-ENRICHED
- Page 287 and 288: NIowLy ENmI ToLow-EmIAEDURAwrum CoN
- Page 289 and 290: Standard Review Plan and Acceptance
- Page 291 and 292: HEU To LEU CoNvEaswoN since the las
- Page 293 and 294: HEU To LEU CONVERSION should also d
- Page 295 and 296: HEU TO LEU CoNVERSION this section,
- Page 297 and 298: 4.2.1 Fuel Elements HEU TO LEU CONV
- Page 299 and 300: Evaluation Findings (Sections 4.2.1
- Page 301 and 302: Acceptance Criteria _HEU TOLEU CoNv
- Page 303 and 304:
- HEU TO LEU CONVERSON Calculations
- Page 305 and 306:
HEU TO LEU CONVERSION should be dis
- Page 307 and 308:
HEU TO LEU CoNvERSoN *Acceptable ch
- Page 309 and 310:
BEU To LEU CoNvEasioN Generally, co
- Page 311 and 312:
Evluation Findings HEU TOLEU CONVER
- Page 313 and 314:
... HEU TO LEU CONVERSION * The coo
- Page 315 and 316:
Accepktace Criteria HEU To LEU CONV
- Page 317 and 318:
9.4 Fuel Element Handling and Stora
- Page 319 and 320:
10 EXPERIMENTAL FACILITIES AND UTIL
- Page 321 and 322:
11.1 Radiation Protection Program A
- Page 323 and 324:
*HEU TOLEU CONVERSION The areas of
- Page 325 and 326:
BEU TO LEU CONVERSION specification
- Page 327 and 328:
- - - - HEU To LEU CoNvmESioN relia
- Page 329 and 330:
HEUrTo LEU Cowv~ptstoN design ofthe
- Page 331 and 332:
BIEU To I.EU Col~vERSoN MHA, the ba
- Page 333 and 334:
HEU To LEU CoNvRiow acceptable assu
- Page 335 and 336:
Review Procedures -- HEU TOLEU CONV
- Page 337 and 338:
13.3.1 Loss-of-Coolant Accident Are
- Page 339 and 340:
HEU To LEU CONVERSION methods and a
- Page 341 and 342:
Evaluation Findings BEU To LEU Comv
- Page 343 and 344:
BEU TO LEU COPvERSION characteristi
- Page 345 and 346:
HEU TOLEU CONVON American National
- Page 347 and 348:
HEU TO LEU CONVERSION U.S. Nuclear