Principles of Plant Genetics and Breeding
Principles of Plant Genetics and Breeding
Principles of Plant Genetics and Breeding
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
BREEDING SORGHUM 513<br />
Kansas State University, Texas A&M University, <strong>and</strong> USDA. These public programs do not produce or sell hybrids, but they<br />
develop parental lines <strong>and</strong> germplasms that are used by private industry in commercial hybrid production. In addition, public<br />
research programs in sorghum conduct research in long-term projects such as the introgression <strong>and</strong> development <strong>of</strong> new<br />
germplasm that may provide useful traits in the future.<br />
The Texas Agricultural Experiment Station (TAES) sorghum breeding program located at Texas A&M University in College<br />
Station, Texas is part <strong>of</strong> a multiproject, multilocation sorghum improvement program supported by the TAES. Sorghum breeders<br />
work in conjunction with plant pathologists, entomologists, <strong>and</strong> grain quality <strong>and</strong> molecular geneticists to create an effective <strong>and</strong><br />
important research <strong>and</strong> application oriented team. In terms <strong>of</strong> the sorghum breeding program at College Station, the breeding<br />
program has several objectives: (i) develop <strong>and</strong> release germplasm <strong>and</strong> parental lines with improved adaptability, yield, quality,<br />
<strong>and</strong> stress resistances; (ii) conduct research that increases our underst<strong>and</strong>ing <strong>and</strong> knowledge <strong>of</strong> sorghum breeding <strong>and</strong> genetics;<br />
<strong>and</strong> (iii) train undergraduate <strong>and</strong> graduate students in plant breeding.<br />
Methodology <strong>of</strong> the TAES sorghum breeding program at College Station<br />
For improved hybrids, new <strong>and</strong> improved parental lines must be developed. First, genetic variability must be developed through<br />
the selection <strong>and</strong> hybridization <strong>of</strong> parent material. This is a crucial step in the process. Usually elite germplasm is crossed to other<br />
material (elite lines, germplasm, genetic stocks) to correct a perceived deficiency in the elite material. For example, if an otherwise<br />
good A/B pair is susceptible to lodging, it will be<br />
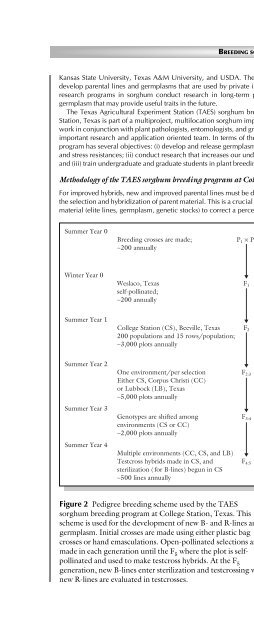
Summer Year 0<br />
Winter Year 0<br />
Summer Year 1<br />
Summer Year 2<br />
Summer Year 3<br />
Summer Year 4<br />
<strong>Breeding</strong> crosses are made;<br />
∼200 annually<br />
Weslaco, Texas<br />
self-pollinated;<br />
∼200 annually<br />
College Station (CS), Beeville, Texas<br />
200 populations <strong>and</strong> 15 rows/population;<br />
∼3,000 plots annually<br />
One environment/per selection<br />
Either CS, Corpus Christi (CC)<br />
or Lubbock (LB), Texas<br />
∼5,000 plots annually<br />
Genotypes are shifted among<br />
environments (CS or CC)<br />
∼2,000 plots annually<br />
Multiple environments (CC, CS, <strong>and</strong> LB)<br />
Testcross hybrids made in CS, <strong>and</strong><br />
sterilization (for B-lines) begun in CS<br />
∼500 lines annually<br />
P 1 × P 2<br />
Figure 2 Pedigree breeding scheme used by the TAES<br />
sorghum breeding program at College Station, Texas. This<br />
scheme is used for the development <strong>of</strong> new B- <strong>and</strong> R-lines <strong>and</strong><br />
germplasm. Initial crosses are made using either plastic bag<br />
crosses or h<strong>and</strong> emasculations. Open-pollinated selections are<br />
made in each generation until the F 5 where the plot is selfpollinated<br />
<strong>and</strong> used to make testcross hybrids. At the F 5<br />
generation, new B-lines enter sterilization <strong>and</strong> testcrossing while<br />
new R-lines are evaluated in testcrosses.<br />
F 1<br />
F 2<br />
F 2:3<br />
F 3:4<br />
F 4:5<br />
hybridized with several different sources <strong>of</strong> lodging<br />
resistance with the goal <strong>of</strong> producing a new A/B pair<br />
with improved lodging resistance. In our program, the<br />
A/B program is managed separately from the R-line<br />
program to maintain heterosis between the two groups<br />
<strong>and</strong> keep the fertility restoration <strong>and</strong> maintenance<br />
genetics separate. Based on the considerations listed<br />
above, specific crosses are made using the methodology<br />
described by Rooney (2004). These F 1 progeny are<br />
self-pollinated to produce an F 2 population.<br />
Once F 2 populations are created, our program utilizes<br />
a pedigree breeding approach for the development<br />
<strong>of</strong> inbred lines (Figure 2). From the F 2 generation<br />
until the F 5 generation (in which uniform lines are<br />
selected), the progeny rows are grown <strong>and</strong> panicles in<br />
the rows are visually selected on the basis <strong>of</strong> agronomic<br />
desirability, pest resistance, <strong>and</strong> abiotic stress<br />
tolerance. F 5 lines that are phenotypically uniform are<br />
testcrossed to measure their general <strong>and</strong> specific combining<br />
ability <strong>and</strong> their suitability as parent lines in<br />
hybrid combinations.<br />
The appropriate time for the selection <strong>of</strong> specific<br />
traits is dependent on the heritability <strong>of</strong> the trait <strong>and</strong><br />
the environments in which the selection occurs. In<br />
our program, traits with higher heritability (maturity,<br />
height, grain color, etc.) are selected in the early generations<br />
while traits with lower heritability (yield,<br />
drought tolerance, disease <strong>and</strong> insect resistance) are<br />
selected in more advanced generations. These more<br />
complexly inherited traits must also be screened in<br />
multiple environments, because these traits may not<br />
be expressed in any given environment. Evaluation in<br />
multiple environments is crucial to the development<br />
<strong>of</strong> widely adapted sorghum genotypes. In our program,<br />
we use three basic regions for inbred selection:<br />
south Texas, central Texas, <strong>and</strong> the Texas high plains<br />
(Figure 3). These regions are each unique <strong>and</strong> force<br />
different selection pressures on the material grown<br />
therein. For example, our south Texas nurseries are<br />
rainfed <strong>and</strong> subject to drought stress <strong>and</strong> consistent<br />
disease pressure. In addition, this region is good for<br />
selecting genotypes that perform well in subtropical