Smithsonian at the Poles: Contributions to International Polar
Smithsonian at the Poles: Contributions to International Polar
Smithsonian at the Poles: Contributions to International Polar
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
face (�1.8°C) are likely <strong>to</strong> pose a strong selective force on<br />
biochemical and molecular function. Understanding how<br />
some organisms have adapted <strong>to</strong> this level of n<strong>at</strong>ural selection<br />
will provide inform<strong>at</strong>ion about <strong>the</strong> essential set of<br />
genetic components necessary for survival <strong>at</strong> low temper<strong>at</strong>ure<br />
margins of our biosphere.<br />
EMBRYOS IN THE COLD<br />
The fact th<strong>at</strong> polar marine invertebr<strong>at</strong>es can maintain<br />
a complex program of embryological development<br />
<strong>at</strong> low temper<strong>at</strong>ures has received much discussion in <strong>the</strong><br />
liter<strong>at</strong>ure in terms of life-his<strong>to</strong>ry adapt<strong>at</strong>ions. Of <strong>the</strong>se<br />
ecological studies, Thorson’s rule has provided a focal<br />
point for numerous consider<strong>at</strong>ions of why development<br />
is so prolonged in marine invertebr<strong>at</strong>es <strong>at</strong> high l<strong>at</strong>itudes<br />
(Pearse et al., 1991; Pearse and Lockhart, 2004). The limited<br />
availability of food in polar oceans has lead <strong>to</strong> uncertainty<br />
regarding <strong>the</strong> rel<strong>at</strong>ive importance of low food<br />
availability compared with low temper<strong>at</strong>ures as <strong>the</strong> primary<br />
selective force limiting developmental r<strong>at</strong>es (Clarke,<br />
1991; Clarke et al., 2007). Overall, limits on metabolism<br />
have now received considerable <strong>at</strong>tention in <strong>the</strong> liter<strong>at</strong>ure<br />
and a general syn<strong>the</strong>sis of <strong>the</strong> physiological constraints on<br />
organismal function in polar environments now appears<br />
<strong>to</strong> primarily involve cellular energetics <strong>at</strong> molecular and<br />
biochemical levels (Peck et al., 2004; Peck et al., 2006a;<br />
Peck et al., 2006b; Clarke et al., 2007). Recent work with<br />
<strong>the</strong> plank<strong>to</strong>trophic larvae of <strong>the</strong> Antarctic sea urchin, Sterechinus<br />
neumayeri, has demonstr<strong>at</strong>ed th<strong>at</strong> changes in <strong>the</strong><br />
nutritional st<strong>at</strong>e of this feeding larvae do not alter its r<strong>at</strong>e<br />
of early larval development (Marsh and Manahan, 1999;<br />
2000). Low temper<strong>at</strong>ures are likely a primary selective<br />
force and <strong>the</strong>se larvae exhibit unique molecular adapt<strong>at</strong>ions<br />
<strong>to</strong> conserve cellular energy (Marsh et al., 2001b).<br />
This may be a general characteristic of polar invertebr<strong>at</strong>e<br />
larvae, and could account for <strong>the</strong> predominance of nonfeeding<br />
developmental modes found among benthic, macrofaunal<br />
invertebr<strong>at</strong>es in Antarctica, particularly among<br />
echinoderms.<br />
EMBRYO ENERGY METABOLISM<br />
One of <strong>the</strong> most striking characteristics of embryonic<br />
development in Antarctic marine invertebr<strong>at</strong>es is <strong>the</strong> slow<br />
r<strong>at</strong>e of cell division. In <strong>the</strong> sea urchin S. neumayeri, early<br />
cleavage has a cell cycle period of 12 h, which is an order<br />
of magnitude slower than in a temper<strong>at</strong>e sea urchin embryo<br />
<strong>at</strong> 15°C. In <strong>the</strong> Antarctic asteroid Odontaster validus <strong>the</strong><br />
COLD ADAPTATION IN POLAR MARINE INVERTEBRATES 255<br />
embryonic cell cycle period is just as long, and in <strong>the</strong> Antarctic<br />
mollusk Tri<strong>to</strong>nia antarctica, it is extended <strong>to</strong> almost<br />
48 h (Marsh, University of Delware, unpublished d<strong>at</strong>a). In<br />
general we assume th<strong>at</strong> cell division is linked or coordin<strong>at</strong>ed<br />
<strong>to</strong> metabolic r<strong>at</strong>es and th<strong>at</strong> <strong>the</strong> increase in cell cycle<br />
period results from an overall decrease in metabolic r<strong>at</strong>e<br />
processing <strong>at</strong> low temper<strong>at</strong>ures. Embryos of S. neumayeri<br />
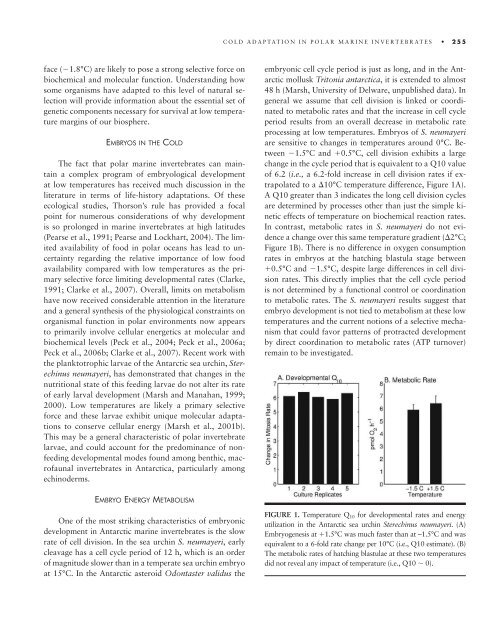
are sensitive <strong>to</strong> changes in temper<strong>at</strong>ures around 0°C. Between<br />
�1.5°C and �0.5°C, cell division exhibits a large<br />
change in <strong>the</strong> cycle period th<strong>at</strong> is equivalent <strong>to</strong> a Q10 value<br />
of 6.2 (i.e., a 6.2-fold increase in cell division r<strong>at</strong>es if extrapol<strong>at</strong>ed<br />
<strong>to</strong> a �10°C temper<strong>at</strong>ure difference, Figure 1A).<br />
A Q10 gre<strong>at</strong>er than 3 indic<strong>at</strong>es <strong>the</strong> long cell division cycles<br />
are determined by processes o<strong>the</strong>r than just <strong>the</strong> simple kinetic<br />
effects of temper<strong>at</strong>ure on biochemical reaction r<strong>at</strong>es.<br />
In contrast, metabolic r<strong>at</strong>es in S. neumayeri do not evidence<br />
a change over this same temper<strong>at</strong>ure gradient (�2°C;<br />
Figure 1B). There is no difference in oxygen consumption<br />
r<strong>at</strong>es in embryos <strong>at</strong> <strong>the</strong> h<strong>at</strong>ching blastula stage between<br />
�0.5°C and �1.5°C, despite large differences in cell division<br />
r<strong>at</strong>es. This directly implies th<strong>at</strong> <strong>the</strong> cell cycle period<br />
is not determined by a functional control or coordin<strong>at</strong>ion<br />
<strong>to</strong> metabolic r<strong>at</strong>es. The S. neumayeri results suggest th<strong>at</strong><br />
embryo development is not tied <strong>to</strong> metabolism <strong>at</strong> <strong>the</strong>se low<br />
temper<strong>at</strong>ures and <strong>the</strong> current notions of a selective mechanism<br />
th<strong>at</strong> could favor p<strong>at</strong>terns of protracted development<br />
by direct coordin<strong>at</strong>ion <strong>to</strong> metabolic r<strong>at</strong>es (ATP turnover)<br />
remain <strong>to</strong> be investig<strong>at</strong>ed.<br />
FIGURE 1. Temper<strong>at</strong>ure Q10 for developmental r<strong>at</strong>es and energy<br />
utiliz<strong>at</strong>ion in <strong>the</strong> Antarctic sea urchin Sterechinus neumayeri. (A)<br />
Embryogenesis <strong>at</strong> �1.5°C was much faster than <strong>at</strong> – 1.5°C and was<br />
equivalent <strong>to</strong> a 6-fold r<strong>at</strong>e change per 10°C (i.e., Q10 estim<strong>at</strong>e). (B)<br />
The metabolic r<strong>at</strong>es of h<strong>at</strong>ching blastulae <strong>at</strong> <strong>the</strong>se two temper<strong>at</strong>ures<br />
did not reveal any impact of temper<strong>at</strong>ure (i.e., Q10 � 0).