- Page 2 and 3:
R. Meyer J. Köhler A. Homburg Expl
- Page 4 and 5:
Dr. Rudolf Meyer (†) (formerly: W
- Page 6 and 7:
Preface and Prof. Dr. P. Elsner, Dr
- Page 8:
From the preface of previous editio
- Page 12 and 13:
mass: kg g oz. Ib. kilogram: 1 kg =
- Page 14 and 15:
1 Adiabatic Abel Test This test on
- Page 16 and 17:
3 Airbag types of generators. Both
- Page 18 and 19:
5 Airbag 8 7 4 3 1 2 5 6 1. Ignitio
- Page 20 and 21:
7 Airbag ates and chlorates with ad
- Page 22 and 23:
9 Akardite III Specifications melti
- Page 24 and 25:
11 Ammongelit 2; 3 Aluminum Powder
- Page 26 and 27:
13 Ammonium Chloride Vapor pressure
- Page 28 and 29:
15 Ammonium Nitrate the most powerf
- Page 30 and 31:
17 Ammonium Perchlorate Higher dens
- Page 32 and 33:
19 Amorces Ammonium Picrate ammoniu
- Page 34 and 35:
21 Armor Plate Impact Test Aquarium
- Page 36 and 37:
23 Average Burning Rate front face
- Page 38 and 39:
25 Ballistic Bomb z(p/pmax) = p/pma
- Page 40 and 41:
27 Ballistic Mortar liveliness at p
- Page 42 and 43:
29 Barium Perchlorate Baratols Pour
- Page 44 and 45:
31 Bengal Fireworks An oxidizer in
- Page 46 and 47:
33 BICT employed, since differing v
- Page 48 and 49:
35 Black Powder and heat of explosi
- Page 50 and 51:
37 Blasting Gelatin agents are cons
- Page 52 and 53:
39 Booster Blasting Switch Zündsch
- Page 54 and 55:
41 Brisance Bridgewire Detonator Br
- Page 56 and 57:
43 Bullet-resistant Bulldoze Aufleg
- Page 58 and 59:
45 Burning Rate Burning Rate Abbran
- Page 60 and 61:
47 Butanetriol Trinitrate Butanedio
- Page 62 and 63:
49 Cap Sensitivity The following da
- Page 64 and 65:
51 Cap Sensitivity and initiated. N
- Page 66 and 67:
53 Case Bonding Table 3. Cartridge
- Page 68 and 69:
55 CDB Propellants Since W TNT is p
- Page 70 and 71:
57 Centralite III Centralite II dim
- Page 72 and 73:
59 Combustibility Class A, Class B
- Page 74 and 75:
61 Composite Propellants the chambe
- Page 76 and 77:
63 Confined Detonation Velocity Com
- Page 78 and 79:
65 Coyote Blasting Copper chromite
- Page 80 and 81:
67 Cutting Charges Curing Härten;
- Page 82 and 83:
69 Cylinder Expansion Test energy o
- Page 84 and 85:
71 Dangerous Goods Regulations Fig.
- Page 86 and 87:
73 Deflagration Point Deckmaster Tr
- Page 88 and 89:
75 Destruction of Explosive Materia
- Page 90 and 91:
77 Detonation fuses for the safe in
- Page 92 and 93:
79 Detonation The state variables w
- Page 94 and 95:
81 Detonation perature and pressure
- Page 96 and 97:
83 Detonation of aggregation. They
- Page 98 and 99:
85 Detonation 4. Sympathetic Detona
- Page 100 and 101:
87 Detonation Fig. 14. Gap test acc
- Page 102 and 103:
89 Detonation 6. Detonation Develop
- Page 104 and 105:
91 1,1-Diamino-2,2-dinitroethylene
- Page 106 and 107:
93 Dibutyl Phthalate at r = 1.5 g/c
- Page 108 and 109:
95 Diethyleneglycol Dinitrate Dieth
- Page 110 and 111:
97 Dimethylhydrazine, unsymmetrical
- Page 112 and 113:
99 4,6-Dinitrobenzofuroxan colorles
- Page 114 and 115:
101 Dinitrodioxyethyloxamide Dinitr
- Page 116 and 117:
103 Dinitroorthocresol molecular we
- Page 118 and 119:
105 Dinitrosobenzene energy of form
- Page 120 and 121:
107 Dinitrotoluene heat of fusion:
- Page 122 and 123:
109 Diphenylamine oxygen balance: -
- Page 124 and 125:
111 Ditching Dynamite Dipicrylurea
- Page 126 and 127:
113 Dynaschoc An advantage of this
- Page 128 and 129:
115 End of Burning Ednatol A cast e
- Page 130 and 131:
117 Energy of Formation; Enthalpy o
- Page 132 and 133:
119 Equation of State the calculati
- Page 134 and 135:
121 Erythritol Tetranitrate Erosion
- Page 136 and 137:
123 Ethylenediamine Dinitrate oxyge
- Page 138 and 139:
125 Ethylphenylurethane Ethyl Nitra
- Page 140 and 141:
127 Exothermal oxygen balance: -61.
- Page 142 and 143:
129 Explosive Forming and Cladding
- Page 144 and 145:
131 Explosives W Class A Explosives
- Page 146 and 147:
133 Explosives W Diethyleneglycol D
- Page 148 and 149:
Table 12. Requirements on Industria
- Page 150 and 151:
137 Firing Current combustion chamb
- Page 152 and 153:
139 Fragmentation Test plosives wit
- Page 154 and 155:
141 Friction Sensitivity Sensitiven
- Page 156 and 157:
143 Fumes may lead to heavy interna
- Page 158 and 159:
145 Gap Test Functioning Time Ignit
- Page 160 and 161:
147 Gelatins; Gelatinous Explosives
- Page 162 and 163:
149 Glycerol - 2,4-Dinitrophenyl Et
- Page 164 and 165:
151 Glycidyl Azide Polymer lead blo
- Page 166 and 167:
153 Guanidine Nitrate Guanidine Nit
- Page 168 and 169:
155 Gunpowder them from influx of w
- Page 170 and 171:
157 Gurney energy which these powde
- Page 172 and 173:
159 Heat of Explosion usually have
- Page 174 and 175:
161 Partial Heat of Explosion Compo
- Page 176 and 177:
163 Heat Sensitivity In this way, r
- Page 178 and 179:
165 Hexamethylene Diisocyanate oxyg
- Page 180 and 181:
167 Hexanitroazobenzene energy of f
- Page 182 and 183:
169 Hexanitrodiphenylaminoethyl Nit
- Page 184 and 185:
171 2,4,6,2',4',6'-Hexanitrodipheny
- Page 186 and 187:
173 Hexanitrooxanilide energy of fo
- Page 188 and 189:
175 Hexogen heat of explosion calcu
- Page 190 and 191:
177 Hot Storage Tests Specification
- Page 192 and 193:
179 Hybrids criterion of the propel
- Page 194 and 195:
181 Hygroscopicity melting point: s
- Page 196 and 197:
183 Illuminant Composition Igniter
- Page 198 and 199:
185 Impact Sensitivity each case. T
- Page 200 and 201:
187 Impact Sensitivity Table 18. Im
- Page 202 and 203:
189 To Inflame Table 20. High criti
- Page 204 and 205:
191 Ion Propellants Non-brisant, i.
- Page 206 and 207:
193 Lambrit impact sensitivity: 1.5
- Page 208 and 209:
195 Lead Azide energy of formation:
- Page 210 and 211:
197 Lead Block Test 25 mm in height
- Page 212 and 213:
199 Lead Nitrate Lead Ethylhexanoat
- Page 214 and 215:
201 Leading Lines heat of explosion
- Page 216 and 217:
203 Liquid Propellants explosive st
- Page 218 and 219:
205 LOVA Gun-Propellant LOVA An abb
- Page 220 and 221:
207 Mannitol Hexanitrate Magazine S
- Page 222 and 223:
209 Mercury Fulminate lightly or he
- Page 224 and 225:
211 Methylamine Nitrate detonation
- Page 226 and 227:
213 Methyl Violet Test Methylphenyl
- Page 228 and 229:
215 Miniaturized Detonating Cord Mi
- Page 230 and 231:
217 Mud Cap Motor Motor; moteur Gen
- Page 232 and 233:
219 Nitrocarbonitrate and the use o
- Page 234 and 235:
221 Nitrocellulose the following da
- Page 236 and 237:
223 Nitroglycerine heat of explosio
- Page 238 and 239:
225 Nitroglycerine cerine produced
- Page 240 and 241:
227 Nitroglycol Nitroglycol ethylen
- Page 242 and 243:
229 Nitroguanidine; Picrite molecul
- Page 244 and 245:
231 Nitromethane detonation velocit
- Page 246 and 247:
233 Nitrostarch (H2O liq.): 1266 kc
- Page 248 and 249:
235 Nitrourea energy of formation:
- Page 250 and 251:
237 Octogen Nozzle Düse; tuyère M
- Page 252 and 253:
239 Oxidizer The compound is formed
- Page 254 and 255:
241 Parallel Connection The most fa
- Page 256 and 257:
243 Pentaerythritol Trinitrate Inje
- Page 258 and 259:
245 Permissibles; Permitted Explosi
- Page 260 and 261:
247 Permissibles; Permitted Explosi
- Page 262 and 263:
249 Permissibles; Permitted Explosi
- Page 264 and 265:
251 PETN vapor pressure: Pressure T
- Page 266 and 267:
253 Picratol Picramic Acid Dinitroa
- Page 268 and 269:
255 Plate Dent Test acid is prepare
- Page 270 and 271:
257 Poly-3,3-bis-(azidomethyl)-oxet
- Page 272 and 273:
259 Polyvinyl Nitrate PPG serves as
- Page 274 and 275:
261 Potassium Perchlorate It is use
- Page 276 and 277:
263 Prequalification Test Powder Fo
- Page 278 and 279:
265 Progressive Burning Powder Prim
- Page 280 and 281:
267 Propyleneglycol Dinitrate Prope
- Page 282 and 283:
269 Quick-Match Pyrophoric Material
- Page 284 and 285:
271 RID Relay An explosive train co
- Page 286 and 287:
273 Rocket Test Stand Rifle Bullet
- Page 288 and 289:
275 Sand Test The black powder cont
- Page 290 and 291:
277 Seismo-Gelit Screw extruders we
- Page 292 and 293:
279 Sensitivity 360 mm wide by 2 mm
- Page 294 and 295:
281 Shaped Charges ner can pierce s
- Page 296 and 297:
283 Silver Azide Shot Firer Sprengm
- Page 298 and 299: 285 SINCO ® Ignition booster and g
- Page 300 and 301: 287 Slurrit Skid Test This test is
- Page 302 and 303: 289 Solid Propellant Rockets The sa
- Page 304 and 305: 291 Specific Energy grain (Bernoull
- Page 306 and 307: 293 Squib Hc: enthalpy of the react
- Page 308 and 309: 295 Stabilizers Stabilizers Stabili
- Page 310 and 311: 297 Strength relevant parameter is
- Page 312 and 313: 299 Strength Fig. 22. Specific ener
- Page 314 and 315: 301 Surface Treatment Styphnic acid
- Page 316 and 317: 303 Tacot Fig. 24. Test results. Sy
- Page 318 and 319: 305 Tetramethylammonium Nitrate ing
- Page 320 and 321: 307 Tetranitrocarbazole 2,3,4,6-Tet
- Page 322 and 323: 309 Tetranitronaphthalene Tetranitr
- Page 324 and 325: 311 Tetryl Tetryl trinitro-2,4,6-ph
- Page 326 and 327: 313 Thermodynamic Calculation of De
- Page 328 and 329: 315 Thermodynamic Calculation of De
- Page 330 and 331: 317 Thermodynamic Calculation of De
- Page 332 and 333: 319 Thermodynamic Calculation of De
- Page 334 and 335: 321 Thermodynamic Calculation of De
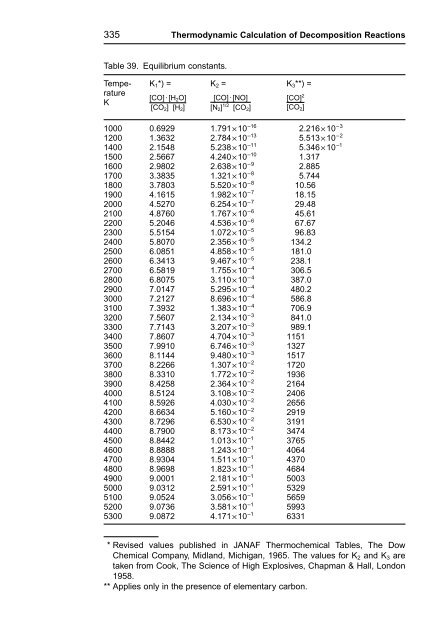
- Page 336 and 337: 323 Thermodynamic Calculation of De
- Page 338 and 339: 325 Thermodynamic Calculation of De
- Page 340 and 341: 327 Thermodynamic Calculation of De
- Page 342 and 343: Table 34. Enthalpy and energy of fo
- Page 344 and 345: 331 Thermodynamic Calculation of De
- Page 346 and 347: 333 Thermodynamic Calculation of De
- Page 350 and 351: 337 TNT heat of explosion (H2O gas)
- Page 352 and 353: 339 Tracers Table 40. Data of the n
- Page 354 and 355: 341 1,3,5-Triamino-2,4,6-trinitrobe
- Page 356 and 357: 343 Triethyleneglycol Dinitrate thu
- Page 358 and 359: 345 Trimethyleneglycol Dinitrate Tr
- Page 360 and 361: 347 1,3,3-Trinitroazetidine oxygen
- Page 362 and 363: 349 Trinitrobenzoic Acid lead block
- Page 364 and 365: 351 2,4,6-Trinitrocresol Pressure T
- Page 366 and 367: 353 Trinitrophenoxethyl nitrate Tri
- Page 368 and 369: 355 Trinitropyridine-N-oxide It is
- Page 370 and 371: 357 Underwater Detonations Trixogen
- Page 372 and 373: 359 Upsetting Tests From the observ
- Page 374 and 375: 361 U-Zünder Urea Nitrate Harnstof
- Page 376 and 377: 363 Volume of Explosion Gases Vibro
- Page 378 and 379: 365 Water Stemming cut off at one e
- Page 380 and 381: 367 Zinc Peroxide X-Ray Flash By us
- Page 382 and 383: 369 Literature Roth, J.F.: Sprengst
- Page 384 and 385: 371 Literature Gustafson, R.: Swedi
- Page 386 and 387: 373 Literature Wiech, R.E. und Stra
- Page 388 and 389: 375 Literature Zeldovich, J.B. und
- Page 390 and 391: 377 Literature Goad, K.J.W. und Arc
- Page 392 and 393: 379 Literature Los Alamos Shock Wav
- Page 394 and 395: 381 Literature Mining and Minerals
- Page 396 and 397: 383 Literature 5. Joint Internation
- Page 398 and 399:
Index aluminum powder 11; 12; 215;
- Page 400 and 401:
Index billet 33 binder 34 binary ex
- Page 402 and 403:
Index Cellulosenitrat W nitrocellul
- Page 404 and 405:
Index danger d’explosion en masse
- Page 406 and 407:
Index Dinol = diazodinitrophenol (g
- Page 408 and 409:
Index essai au bloc de plomb = lead
- Page 410 and 411:
Index fracture, épreuve de 139 fra
- Page 412 and 413:
Index HC = mixture of hexachloroeth
- Page 414 and 415:
Index IME = Institute of Makers of
- Page 416 and 417:
Index Lebhaftigkeitsfaktor = vivaci
- Page 418 and 419:
Index MNM = mononitromethane 231 M.
- Page 420 and 421:
Index Normalgasvolumen = Normalvolu
- Page 422 and 423:
Index plane wave generators = charg
- Page 424 and 425:
Index raté = misfire 216 RATO = ro
- Page 426 and 427:
Index SFF = EFP 281 S.G.P. = denomi
- Page 428 and 429:
Index TDI = toluene diisocyanate 33
- Page 430 and 431:
Index TPEON = tripentaerythroloctan
- Page 432 and 433:
Index W W I; W II; W III = permitte