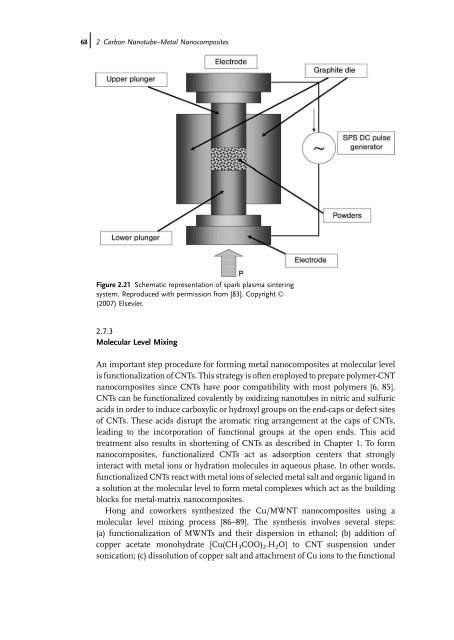
- Page 2 and 3:
Sie Chin Tjong Carbon Nanotube Rein
- Page 4 and 5:
Sie Chin Tjong Carbon Nanotube Rein
- Page 6 and 7:
Contents Preface IX List of Abbrevi
- Page 8 and 9:
5 Carbon Nanotube-Ceramic Nanocompo
- Page 10:
Preface Carbon nanotubes are nanost
- Page 13 and 14:
XII List of Abbreviations HIP hot i
- Page 16 and 17:
1 Introduction 1.1 Background Compo
- Page 18 and 19:
Figure 1.2 Transmission electron mi
- Page 20 and 21:
1.3 Synthesis of Carbon Nanotubes 1
- Page 22 and 23:
MWNTs can be as high as 70% of the
- Page 24 and 25:
Figure 1.7 In situ TEM images recor
- Page 26 and 27:
1.3 Synthesis of Carbon Nanotubesj1
- Page 28 and 29:
show TEM images of MWNTs synthesize
- Page 30 and 31:
Since then, large efforts have been
- Page 32 and 33: 1.3.4 Patent Processes Carbon nanot
- Page 34 and 35: 1.4 Purification of Carbon Nanotube
- Page 36 and 37: Table 1.3 Patent processes for the
- Page 38 and 39: Figure 1.13 Schematic illustration
- Page 40 and 41: 1.5 Mechanical Properties of Carbon
- Page 42 and 43: Figure 1.14 Carbon nanotubes in hig
- Page 44 and 45: Figure 1.15 In situ tensile deforma
- Page 46 and 47: Table 1.7 Theoretical and experimen
- Page 48 and 49: Nomenclature ~a 1 , ~a 2 Unit vecto
- Page 50 and 51: carbon nanotubes. Physical Review L
- Page 52 and 53: 70 Jang, I., Uh, H.S., Cho, H.J., L
- Page 54 and 55: 108 Shelimov, K.B., Esenaliev, R.O.
- Page 56 and 57: Bonnamy, S., Beguin, F., Burnham, N
- Page 58 and 59: 2 Carbon Nanotube-Metal Nanocomposi
- Page 60 and 61: isostatic pressing. In certain case
- Page 62 and 63: This implies the absence of effecti
- Page 64 and 65: coating material, the plasma gun an
- Page 66 and 67: Table 2.4 Changes in the size and v
- Page 68 and 69: Figure 2.6 (a) Low and (b) high mag
- Page 70 and 71: Figure 2.8 SEM micrographs showing
- Page 72 and 73: Figure 2.11 Bright field TEM microg
- Page 74 and 75: Figure 2.13 TEM image of bulk Al/5
- Page 76 and 77: 2.5 Magnesium-Based Nanocomposites
- Page 78 and 79: limited improvement in ultimate ten
- Page 80 and 81: Figure 2.18 SEM micrographs of the
- Page 84 and 85: Figure 2.22 SEM micrographs of (a)
- Page 86 and 87: Figure 2.25 (a) TEM micrograph show
- Page 88 and 89: 2.8 Transition Metal-Based Nanocomp
- Page 90 and 91: Figure 2.27 TEM image of electrodep
- Page 92 and 93: Figure 2.29 SEM micrographs of (a)
- Page 94 and 95: Figure 2.31 Schematic representatio
- Page 96 and 97: einforcement content SiCp/Al compos
- Page 98 and 99: Materials Science Forum, 534-536 (P
- Page 100 and 101: composite. Materials Science and En
- Page 102: 111 Cha, S.I., Kim, K.T., Arshad, S
- Page 105 and 106: 90j 3 Physical Properties of Carbon
- Page 107 and 108: 92j 3 Physical Properties of Carbon
- Page 109 and 110: 94j 3 Physical Properties of Carbon
- Page 111 and 112: 96j 3 Physical Properties of Carbon
- Page 113 and 114: 98j 3 Physical Properties of Carbon
- Page 115 and 116: 100j 3 Physical Properties of Carbo
- Page 117 and 118: 102j 3 Physical Properties of Carbo
- Page 119 and 120: 104j 4 Mechanical Characteristics o
- Page 121 and 122: 106j 4 Mechanical Characteristics o
- Page 123 and 124: 108j 4 Mechanical Characteristics o
- Page 125 and 126: 110j 4 Mechanical Characteristics o
- Page 127 and 128: 112j 4 Mechanical Characteristics o
- Page 129 and 130: 114j 4 Mechanical Characteristics o
- Page 131 and 132: 116j 4 Mechanical Characteristics o
- Page 133 and 134:
118j 4 Mechanical Characteristics o
- Page 135 and 136:
120j 4 Mechanical Characteristics o
- Page 137 and 138:
122j 4 Mechanical Characteristics o
- Page 139 and 140:
124j 4 Mechanical Characteristics o
- Page 141 and 142:
126j 4 Mechanical Characteristics o
- Page 143 and 144:
128j 4 Mechanical Characteristics o
- Page 145 and 146:
130j 4 Mechanical Characteristics o
- Page 147 and 148:
132j 5 Carbon Nanotube-Ceramic Nano
- Page 149 and 150:
134j 5 Carbon Nanotube-Ceramic Nano
- Page 151 and 152:
136j 5 Carbon Nanotube-Ceramic Nano
- Page 153 and 154:
138j 5 Carbon Nanotube-Ceramic Nano
- Page 155 and 156:
140j 5 Carbon Nanotube-Ceramic Nano
- Page 157 and 158:
142j 5 Carbon Nanotube-Ceramic Nano
- Page 159 and 160:
144j 5 Carbon Nanotube-Ceramic Nano
- Page 161 and 162:
146j 5 Carbon Nanotube-Ceramic Nano
- Page 163 and 164:
148j 5 Carbon Nanotube-Ceramic Nano
- Page 165 and 166:
150j 5 Carbon Nanotube-Ceramic Nano
- Page 167 and 168:
152j 5 Carbon Nanotube-Ceramic Nano
- Page 169 and 170:
154j 5 Carbon Nanotube-Ceramic Nano
- Page 171 and 172:
156j 5 Carbon Nanotube-Ceramic Nano
- Page 173 and 174:
158j 5 Carbon Nanotube-Ceramic Nano
- Page 175 and 176:
160j 5 Carbon Nanotube-Ceramic Nano
- Page 177 and 178:
162j 5 Carbon Nanotube-Ceramic Nano
- Page 179 and 180:
164j 5 Carbon Nanotube-Ceramic Nano
- Page 181 and 182:
166j 5 Carbon Nanotube-Ceramic Nano
- Page 183 and 184:
168j 5 Carbon Nanotube-Ceramic Nano
- Page 185 and 186:
170j 6 Physical Properties of Carbo
- Page 187 and 188:
172j 6 Physical Properties of Carbo
- Page 189 and 190:
174j 6 Physical Properties of Carbo
- Page 191 and 192:
176j 6 Physical Properties of Carbo
- Page 193 and 194:
178j 6 Physical Properties of Carbo
- Page 195 and 196:
180j 6 Physical Properties of Carbo
- Page 197 and 198:
182j 6 Physical Properties of Carbo
- Page 199 and 200:
184j 6 Physical Properties of Carbo
- Page 201 and 202:
186j 7 Mechanical Properties of Car
- Page 203 and 204:
188j 7 Mechanical Properties of Car
- Page 205 and 206:
190j 7 Mechanical Properties of Car
- Page 207 and 208:
192j 7 Mechanical Properties of Car
- Page 209 and 210:
194j 7 Mechanical Properties of Car
- Page 211 and 212:
196j 7 Mechanical Properties of Car
- Page 213 and 214:
198j 7 Mechanical Properties of Car
- Page 215 and 216:
200j 7 Mechanical Properties of Car
- Page 217 and 218:
202j 7 Mechanical Properties of Car
- Page 219 and 220:
204j 7 Mechanical Properties of Car
- Page 221 and 222:
206j 7 Mechanical Properties of Car
- Page 223 and 224:
208j 7 Mechanical Properties of Car
- Page 225 and 226:
210j 7 Mechanical Properties of Car
- Page 227 and 228:
212j 7 Mechanical Properties of Car
- Page 229 and 230:
214j 7 Mechanical Properties of Car
- Page 231 and 232:
Table 8.1 Patent processes for maki
- Page 233 and 234:
218j 8 Conclusions development of c
- Page 235 and 236:
220j 8 Conclusions Figure 8.2 TEM m
- Page 237 and 238:
222j 8 Conclusions increase in Vick
- Page 239 and 240:
224j 8 Conclusions References 1 Cha
- Page 241 and 242:
226j 8 Conclusions nano-matrix. Scr
- Page 243:
228j Index l laser ablation 7 load