Reproduction in Domestic Animals
Reproduction in Domestic Animals
Reproduction in Domestic Animals
- No tags were found...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
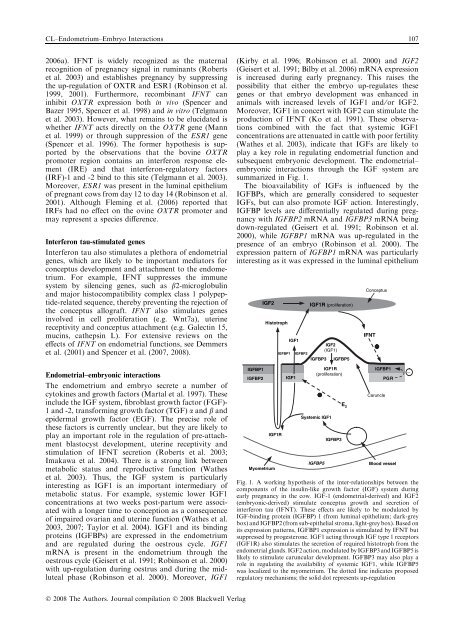
CL–Endometrium–Embryo Interactions 1072006a). IFNT is widely recognized as the maternalrecognition of pregnancy signal <strong>in</strong> rum<strong>in</strong>ants (Robertset al. 2003) and establishes pregnancy by suppress<strong>in</strong>gthe up-regulation of OXTR and ESR1 (Rob<strong>in</strong>son et al.1999, 2001). Furthermore, recomb<strong>in</strong>ant IFNT can<strong>in</strong>hibit OXTR expression both <strong>in</strong> vivo (Spencer andBazer 1995, Spencer et al. 1998) and <strong>in</strong> vitro (Telgmannet al. 2003). However, what rema<strong>in</strong>s to be elucidated iswhether IFNT acts directly on the OXTR gene (Mannet al. 1999) or through suppression of the ESR1 gene(Spencer et al. 1996). The former hypothesis is supportedby the observations that the bov<strong>in</strong>e OXTRpromoter region conta<strong>in</strong>s an <strong>in</strong>terferon response element(IRE) and that <strong>in</strong>terferon-regulatory factors(IRF)-1 and -2 b<strong>in</strong>d to this site (Telgmann et al. 2003).Moreover, ESR1 was present <strong>in</strong> the lum<strong>in</strong>al epitheliumof pregnant cows from day 12 to day 14 (Rob<strong>in</strong>son et al.2001). Although Flem<strong>in</strong>g et al. (2006) reported thatIRFs had no effect on the ov<strong>in</strong>e OXTR promoter andmay represent a species difference.Interferon tau-stimulated genesInterferon tau also stimulates a plethora of endometrialgenes, which are likely to be important mediators forconceptus development and attachment to the endometrium.For example, IFNT suppresses the immunesystem by silenc<strong>in</strong>g genes, such as b2-microglobul<strong>in</strong>and major histocompatibility complex class 1 polypeptide-relatedsequence, thereby prevent<strong>in</strong>g the rejection ofthe conceptus allograft. IFNT also stimulates genes<strong>in</strong>volved <strong>in</strong> cell proliferation (e.g. Wnt7a), uter<strong>in</strong>ereceptivity and conceptus attachment (e.g. Galect<strong>in</strong> 15,muc<strong>in</strong>s, catheps<strong>in</strong> L). For extensive reviews on theeffects of IFNT on endometrial functions, see Demmerset al. (2001) and Spencer et al. (2007, 2008).Endometrial–embryonic <strong>in</strong>teractionsThe endometrium and embryo secrete a number ofcytok<strong>in</strong>es and growth factors (Martal et al. 1997). These<strong>in</strong>clude the IGF system, fibroblast growth factor (FGF)-1 and -2, transform<strong>in</strong>g growth factor (TGF) a and b andepidermal growth factor (EGF). The precise role ofthese factors is currently unclear, but they are likely toplay an important role <strong>in</strong> the regulation of pre-attachmentblastocyst development, uter<strong>in</strong>e receptivity andstimulation of IFNT secretion (Roberts et al. 2003;Imakawa et al. 2004). There is a strong l<strong>in</strong>k betweenmetabolic status and reproductive function (Watheset al. 2003). Thus, the IGF system is particularly<strong>in</strong>terest<strong>in</strong>g as IGF1 is an important <strong>in</strong>termediary ofmetabolic status. For example, systemic lower IGF1concentrations at two weeks post-partum were associatedwith a longer time to conception as a consequenceof impaired ovarian and uter<strong>in</strong>e function (Wathes et al.2003, 2007; Taylor et al. 2004). IGF1 and its b<strong>in</strong>d<strong>in</strong>gprote<strong>in</strong>s (IGFBPs) are expressed <strong>in</strong> the endometriumand are regulated dur<strong>in</strong>g the oestrous cycle. IGF1mRNA is present <strong>in</strong> the endometrium through theoestrous cycle (Geisert et al. 1991; Rob<strong>in</strong>son et al. 2000)with up-regulation dur<strong>in</strong>g oestrus and dur<strong>in</strong>g the midlutealphase (Rob<strong>in</strong>son et al. 2000). Moreover, IGF1(Kirby et al. 1996; Rob<strong>in</strong>son et al. 2000) and IGF2(Geisert et al. 1991; Bilby et al. 2006) mRNA expressionis <strong>in</strong>creased dur<strong>in</strong>g early pregnancy. This raises thepossibility that either the embryo up-regulates thesegenes or that embryo development was enhanced <strong>in</strong>animals with <strong>in</strong>creased levels of IGF1 and ⁄ or IGF2.Moreover, IGF1 <strong>in</strong> concert with IGF2 can stimulate theproduction of IFNT (Ko et al. 1991). These observationscomb<strong>in</strong>ed with the fact that systemic IGF1concentrations are attenuated <strong>in</strong> cattle with poor fertility(Wathes et al. 2003), <strong>in</strong>dicate that IGFs are likely toplay a key role <strong>in</strong> regulat<strong>in</strong>g endometrial function andsubsequent embryonic development. The endometrial–embryonic <strong>in</strong>teractions through the IGF system aresummarized <strong>in</strong> Fig. 1.The bioavailability of IGFs is <strong>in</strong>fluenced by theIGFBPs, which are generally considered to sequesterIGFs, but can also promote IGF action. Interest<strong>in</strong>gly,IGFBP levels are differentially regulated dur<strong>in</strong>g pregnancywith IGFBP2 mRNA and IGFBP3 mRNA be<strong>in</strong>gdown-regulated (Geisert et al. 1991; Rob<strong>in</strong>son et al.2000), while IGFBP1 mRNA was up-regulated <strong>in</strong> thepresence of an embryo (Rob<strong>in</strong>son et al. 2000). Theexpression pattern of IGFBP1 mRNA was particularly<strong>in</strong>terest<strong>in</strong>g as it was expressed <strong>in</strong> the lum<strong>in</strong>al epitheliumIGFBP1IGFBP2IGF2MyometriumHistotrophIGF1RIGF1R (proliferation)IGF1IGF2(IGF1)IGFBP1 IGFBP2IGFBP3 IGFBP5IGF1R(proliferation)IGF1Systemic IGF1IGFBP5IGFBP3E 2ConceptusIFNTIGFBP1PGRCaruncleBlood vesselFig. 1. A work<strong>in</strong>g hypothesis of the <strong>in</strong>ter-relationships between thecomponents of the <strong>in</strong>sul<strong>in</strong>-like growth factor (IGF) system dur<strong>in</strong>gearly pregnancy <strong>in</strong> the cow. IGF-1 (endometrial-derived) and IGF2(embryonic-derived) stimulate conceptus growth and secretion of<strong>in</strong>terferon tau (IFNT). These effects are likely to be modulated byIGF-b<strong>in</strong>d<strong>in</strong>g prote<strong>in</strong> (IGFBP) 1 (from lum<strong>in</strong>al epithelium; dark-greybox) and IGFBP2 (from sub-epithelial stroma, light-grey box). Based onits expression patterns, IGFBP1 expression is stimulated by IFNT butsuppressed by progesterone. IGF1 act<strong>in</strong>g through IGF type 1 receptors(IGF1R) also stimulates the secretion of required histotroph from theendometrial glands. IGF2 action, modulated by IGFBP3 and IGFBP5 islikely to stimulate caruncular development. IGFBP3 may also play arole <strong>in</strong> regulat<strong>in</strong>g the availability of systemic IGF1, while IGFBP5was localized to the myometrium. The dotted l<strong>in</strong>e <strong>in</strong>dicates proposedregulatory mechanisms; the solid dot represents up-regulation_Ó 2008 The Authors. Journal compilation Ó 2008 Blackwell Verlag