Reproduction in Domestic Animals
Reproduction in Domestic Animals
Reproduction in Domestic Animals
- No tags were found...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
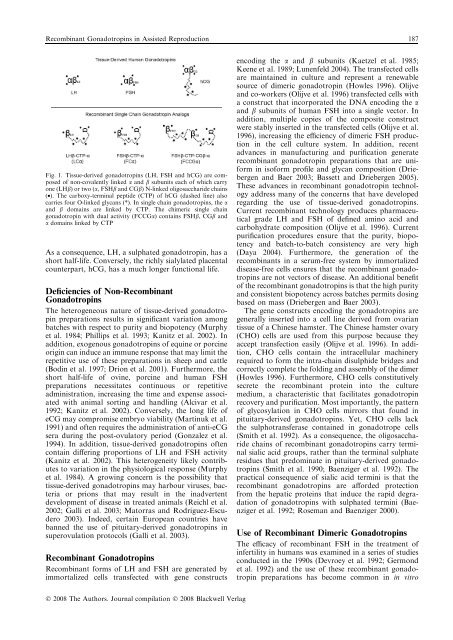
Recomb<strong>in</strong>ant Gonadotrop<strong>in</strong>s <strong>in</strong> Assisted <strong>Reproduction</strong> 187Fig. 1. Tissue-derived gonadotrop<strong>in</strong>s (LH, FSH and hCG) are composedof non-covalently l<strong>in</strong>ked a and b subunits each of which carryone (LHb) or two (a, FSHb and CGb) N-l<strong>in</strong>ked oligosaccharide cha<strong>in</strong>s(•). The carboxy-term<strong>in</strong>al peptide (CTP) of hCG (dashed l<strong>in</strong>e) alsocarries four O-l<strong>in</strong>ked glycans (*). In s<strong>in</strong>gle cha<strong>in</strong> gonadotrop<strong>in</strong>s, the aand b doma<strong>in</strong>s are l<strong>in</strong>ked by CTP. The chimeric s<strong>in</strong>gle cha<strong>in</strong>gonadotrop<strong>in</strong> with dual activity (FCCGa) conta<strong>in</strong>s FSHb, CGb anda doma<strong>in</strong>s l<strong>in</strong>ked by CTPAs a consequence, LH, a sulphated gonadotrop<strong>in</strong>, has ashort half-life. Conversely, the richly sialylated placentalcounterpart, hCG, has a much longer functional life.Deficiencies of Non-Recomb<strong>in</strong>antGonadotrop<strong>in</strong>sThe heterogeneous nature of tissue-derived gonadotrop<strong>in</strong>preparations results <strong>in</strong> significant variation amongbatches with respect to purity and biopotency (Murphyet al. 1984; Phillips et al. 1993; Kanitz et al. 2002). Inaddition, exogenous gonadotrop<strong>in</strong>s of equ<strong>in</strong>e or porc<strong>in</strong>eorig<strong>in</strong> can <strong>in</strong>duce an immune response that may limit therepetitive use of these preparations <strong>in</strong> sheep and cattle(Bod<strong>in</strong> et al. 1997; Drion et al. 2001). Furthermore, theshort half-life of ov<strong>in</strong>e, porc<strong>in</strong>e and human FSHpreparations necessitates cont<strong>in</strong>uous or repetitiveadm<strong>in</strong>istration, <strong>in</strong>creas<strong>in</strong>g the time and expense associatedwith animal sort<strong>in</strong>g and handl<strong>in</strong>g (Alcivar et al.1992; Kanitz et al. 2002). Conversely, the long life ofeCG may compromise embryo viability (Mart<strong>in</strong>uk et al.1991) and often requires the adm<strong>in</strong>istration of anti-eCGsera dur<strong>in</strong>g the post-ovulatory period (Gonzalez et al.1994). In addition, tissue-derived gonadotrop<strong>in</strong>s oftenconta<strong>in</strong> differ<strong>in</strong>g proportions of LH and FSH activity(Kanitz et al. 2002). This heterogeneity likely contributesto variation <strong>in</strong> the physiological response (Murphyet al. 1984). A grow<strong>in</strong>g concern is the possibility thattissue-derived gonadotrop<strong>in</strong>s may harbour viruses, bacteriaor prions that may result <strong>in</strong> the <strong>in</strong>advertentdevelopment of disease <strong>in</strong> treated animals (Reichl et al.2002; Galli et al. 2003; Matorras and Rodriguez-Escudero2003). Indeed, certa<strong>in</strong> European countries havebanned the use of pituitary-derived gonadotrop<strong>in</strong>s <strong>in</strong>superovulation protocols (Galli et al. 2003).Recomb<strong>in</strong>ant Gonadotrop<strong>in</strong>sRecomb<strong>in</strong>ant forms of LH and FSH are generated byimmortalized cells transfected with gene constructsencod<strong>in</strong>g the a and b subunits (Kaetzel et al. 1985;Keene et al. 1989; Lunenfeld 2004). The transfected cellsare ma<strong>in</strong>ta<strong>in</strong>ed <strong>in</strong> culture and represent a renewablesource of dimeric gonadotrop<strong>in</strong> (Howles 1996). Olijveand co-workers (Olijve et al. 1996) transfected cells witha construct that <strong>in</strong>corporated the DNA encod<strong>in</strong>g the aand b subunits of human FSH <strong>in</strong>to a s<strong>in</strong>gle vector. Inaddition, multiple copies of the composite constructwere stably <strong>in</strong>serted <strong>in</strong> the transfected cells (Olijve et al.1996), <strong>in</strong>creas<strong>in</strong>g the efficiency of dimeric FSH production<strong>in</strong> the cell culture system. In addition, recentadvances <strong>in</strong> manufactur<strong>in</strong>g and purification generaterecomb<strong>in</strong>ant gonadotrop<strong>in</strong> preparations that are uniform<strong>in</strong> isoform profile and glycan composition (Driebergenand Baer 2003; Bassett and Driebergen 2005).These advances <strong>in</strong> recomb<strong>in</strong>ant gonadotrop<strong>in</strong> technologyaddress many of the concerns that have developedregard<strong>in</strong>g the use of tissue-derived gonadotrop<strong>in</strong>s.Current recomb<strong>in</strong>ant technology produces pharmaceuticalgrade LH and FSH of def<strong>in</strong>ed am<strong>in</strong>o acid andcarbohydrate composition (Olijve et al. 1996). Currentpurification procedures ensure that the purity, biopotencyand batch-to-batch consistency are very high(Daya 2004). Furthermore, the generation of therecomb<strong>in</strong>ants <strong>in</strong> a serum-free system by immortalizeddisease-free cells ensures that the recomb<strong>in</strong>ant gonadotrop<strong>in</strong>sare not vectors of disease. An additional benefitof the recomb<strong>in</strong>ant gonadotrop<strong>in</strong>s is that the high purityand consistent biopotency across batches permits dos<strong>in</strong>gbased on mass (Driebergen and Baer 2003).The gene constructs encod<strong>in</strong>g the gonadotrop<strong>in</strong>s aregenerally <strong>in</strong>serted <strong>in</strong>to a cell l<strong>in</strong>e derived from ovariantissue of a Ch<strong>in</strong>ese hamster. The Ch<strong>in</strong>ese hamster ovary(CHO) cells are used from this purpose because theyaccept transfection easily (Olijve et al. 1996). In addition,CHO cells conta<strong>in</strong> the <strong>in</strong>tracellular mach<strong>in</strong>eryrequired to form the <strong>in</strong>tra-cha<strong>in</strong> disulphide bridges andcorrectly complete the fold<strong>in</strong>g and assembly of the dimer(Howles 1996). Furthermore, CHO cells constitutivelysecrete the recomb<strong>in</strong>ant prote<strong>in</strong> <strong>in</strong>to the culturemedium, a characteristic that facilitates gonadotrop<strong>in</strong>recovery and purification. Most importantly, the patternof glycosylation <strong>in</strong> CHO cells mirrors that found <strong>in</strong>pituitary-derived gonadotrop<strong>in</strong>s. Yet, CHO cells lackthe sulphotransferase conta<strong>in</strong>ed <strong>in</strong> gonadotrope cells(Smith et al. 1992). As a consequence, the oligosaccharidecha<strong>in</strong>s of recomb<strong>in</strong>ant gonadotrop<strong>in</strong>s carry term<strong>in</strong>alsialic acid groups, rather than the term<strong>in</strong>al sulphateresidues that predom<strong>in</strong>ate <strong>in</strong> pituitary-derived gonadotrop<strong>in</strong>s(Smith et al. 1990; Baenziger et al. 1992). Thepractical consequence of sialic acid term<strong>in</strong>i is that therecomb<strong>in</strong>ant gonadotrop<strong>in</strong>s are afforded protectionfrom the hepatic prote<strong>in</strong>s that <strong>in</strong>duce the rapid degradationof gonadotrop<strong>in</strong>s with sulphated term<strong>in</strong>i (Baenzigeret al. 1992; Roseman and Baenziger 2000).Use of Recomb<strong>in</strong>ant Dimeric Gonadotrop<strong>in</strong>sThe efficacy of recomb<strong>in</strong>ant FSH <strong>in</strong> the treatment of<strong>in</strong>fertility <strong>in</strong> humans was exam<strong>in</strong>ed <strong>in</strong> a series of studiesconducted <strong>in</strong> the 1990s (Devroey et al. 1992; Germondet al. 1992) and the use of these recomb<strong>in</strong>ant gonadotrop<strong>in</strong>preparations has become common <strong>in</strong> <strong>in</strong> vitroÓ 2008 The Authors. Journal compilation Ó 2008 Blackwell Verlag