Reproduction in Domestic Animals
Reproduction in Domestic Animals
Reproduction in Domestic Animals
- No tags were found...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
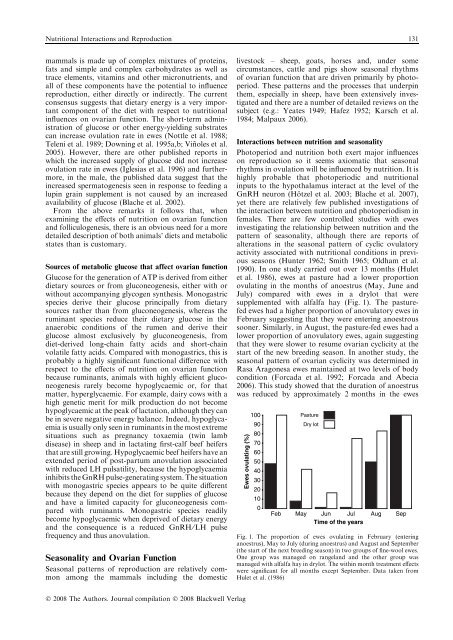
Nutritional Interactions and <strong>Reproduction</strong> 131mammals is made up of complex mixtures of prote<strong>in</strong>s,fats and simple and complex carbohydrates as well astrace elements, vitam<strong>in</strong>s and other micronutrients, andall of these components have the potential to <strong>in</strong>fluencereproduction, either directly or <strong>in</strong>directly. The currentconsensus suggests that dietary energy is a very importantcomponent of the diet with respect to nutritional<strong>in</strong>fluences on ovarian function. The short-term adm<strong>in</strong>istrationof glucose or other energy-yield<strong>in</strong>g substratescan <strong>in</strong>crease ovulation rate <strong>in</strong> ewes (Nottle et al. 1988;Teleni et al. 1989; Down<strong>in</strong>g et al. 1995a,b; V<strong>in</strong>˜ oles et al.2005). However, there are other published reports <strong>in</strong>which the <strong>in</strong>creased supply of glucose did not <strong>in</strong>creaseovulation rate <strong>in</strong> ewes (Iglesias et al. 1996) and furthermore,<strong>in</strong> the male, the published data suggest that the<strong>in</strong>creased spermatogenesis seen <strong>in</strong> response to feed<strong>in</strong>g alup<strong>in</strong> gra<strong>in</strong> supplement is not caused by an <strong>in</strong>creasedavailability of glucose (Blache et al. 2002).From the above remarks it follows that, whenexam<strong>in</strong><strong>in</strong>g the effects of nutrition on ovarian functionand folliculogenesis, there is an obvious need for a moredetailed description of both animals’ diets and metabolicstates than is customary.Sources of metabolic glucose that affect ovarian functionGlucose for the generation of ATP is derived from eitherdietary sources or from gluconeogenesis, either with orwithout accompany<strong>in</strong>g glycogen synthesis. Monogastricspecies derive their glucose pr<strong>in</strong>cipally from dietarysources rather than from gluconeogenesis, whereas therum<strong>in</strong>ant species reduce their dietary glucose <strong>in</strong> theanaerobic conditions of the rumen and derive theirglucose almost exclusively by gluconeogenesis, fromdiet-derived long-cha<strong>in</strong> fatty acids and short-cha<strong>in</strong>volatile fatty acids. Compared with monogastrics, this isprobably a highly significant functional difference withrespect to the effects of nutrition on ovarian functionbecause rum<strong>in</strong>ants, animals with highly efficient gluconeogenesisrarely become hypoglycaemic or, for thatmatter, hyperglycaemic. For example, dairy cows with ahigh genetic merit for milk production do not becomehypoglycaemic at the peak of lactation, although they canbe <strong>in</strong> severe negative energy balance. Indeed, hypoglycaemiais usually only seen <strong>in</strong> rum<strong>in</strong>ants <strong>in</strong> the most extremesituations such as pregnancy toxaemia (tw<strong>in</strong> lambdisease) <strong>in</strong> sheep and <strong>in</strong> lactat<strong>in</strong>g first-calf beef heifersthat are still grow<strong>in</strong>g. Hypoglycaemic beef heifers have anextended period of post-partum anovulation associatedwith reduced LH pulsatility, because the hypoglycaemia<strong>in</strong>hibits the GnRH pulse-generat<strong>in</strong>g system. The situationwith monogastric species appears to be quite differentbecause they depend on the diet for supplies of glucoseand have a limited capacity for gluconeogenesis comparedwith rum<strong>in</strong>ants. Monogastric species readilybecome hypoglycaemic when deprived of dietary energyand the consequence is a reduced GnRH ⁄ LH pulsefrequency and thus anovulation.Seasonality and Ovarian FunctionSeasonal patterns of reproduction are relatively commonamong the mammals <strong>in</strong>clud<strong>in</strong>g the domesticlivestock – sheep, goats, horses and, under somecircumstances, cattle and pigs show seasonal rhythmsof ovarian function that are driven primarily by photoperiod.These patterns and the processes that underp<strong>in</strong>them, especially <strong>in</strong> sheep, have been extensively <strong>in</strong>vestigatedand there are a number of detailed reviews on thesubject (e.g.: Yeates 1949; Hafez 1952; Karsch et al.1984; Malpaux 2006).Interactions between nutrition and seasonalityPhotoperiod and nutrition both exert major <strong>in</strong>fluenceson reproduction so it seems axiomatic that seasonalrhythms <strong>in</strong> ovulation will be <strong>in</strong>fluenced by nutrition. It ishighly probable that photoperiodic and nutritional<strong>in</strong>puts to the hypothalamus <strong>in</strong>teract at the level of theGnRH neuron (Ho¨ tzel et al. 2003; Blache et al. 2007),yet there are relatively few published <strong>in</strong>vestigations ofthe <strong>in</strong>teraction between nutrition and photoperiodism <strong>in</strong>females. There are few controlled studies with ewes<strong>in</strong>vestigat<strong>in</strong>g the relationship between nutrition and thepattern of seasonality, although there are reports ofalterations <strong>in</strong> the seasonal pattern of cyclic ovulatoryactivity associated with nutritional conditions <strong>in</strong> previousseasons (Hunter 1962; Smith 1965; Oldham et al.1990). In one study carried out over 13 months (Huletet al. 1986), ewes at pasture had a lower proportionovulat<strong>in</strong>g <strong>in</strong> the months of anoestrus (May, June andJuly) compared with ewes <strong>in</strong> a drylot that weresupplemented with alfalfa hay (Fig. 1). The pasturefedewes had a higher proportion of anovulatory ewes <strong>in</strong>February suggest<strong>in</strong>g that they were enter<strong>in</strong>g anoestroussooner. Similarly, <strong>in</strong> August, the pasture-fed ewes had alower proportion of anovulatory ewes, aga<strong>in</strong> suggest<strong>in</strong>gthat they were slower to resume ovarian cyclicity at thestart of the new breed<strong>in</strong>g season. In another study, theseasonal pattern of ovarian cyclicity was determ<strong>in</strong>ed <strong>in</strong>Rasa Aragonesa ewes ma<strong>in</strong>ta<strong>in</strong>ed at two levels of bodycondition (Forcada et al. 1992; Forcada and Abecia2006). This study showed that the duration of anoestruswas reduced by approximately 2 months <strong>in</strong> the ewesEwes ovulat<strong>in</strong>g (%)1009080706050403020100FebPastureDry lotMayJun JulTime of the yearsAugSepFig. 1. The proportion of ewes ovulat<strong>in</strong>g <strong>in</strong> February (enter<strong>in</strong>ganoestrus), May to July (dur<strong>in</strong>g anoestrus) and August and September(the start of the next breed<strong>in</strong>g season) <strong>in</strong> two groups of f<strong>in</strong>e-wool ewes.One group was managed on rangeland and the other group wasmanaged with alfalfa hay <strong>in</strong> drylot. The with<strong>in</strong> month treatment effectswere significant for all months except September. Data taken fromHulet et al. (1986)Ó 2008 The Authors. Journal compilation Ó 2008 Blackwell Verlag