Reproduction in Domestic Animals
Reproduction in Domestic Animals
Reproduction in Domestic Animals
- No tags were found...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
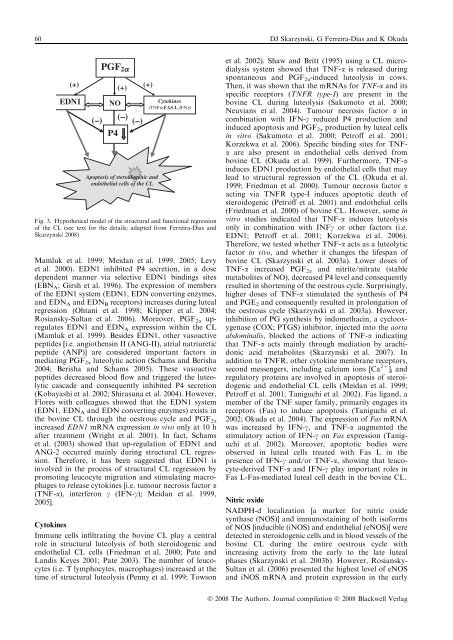
60 DJ Skarzynski, G Ferreira-Dias and K Okuda(+)(+)(+)EDN1(–)PGF 2aNO(–)(–)P4Apoptosis of steroidogenic andendothelial cells of the CLCytok<strong>in</strong>es(TNFa,FAS-L,IFNg)Fig. 3. Hypothetical model of the structural and functional regressionof the CL (see text for the details; adapted from Ferreira-Dias andSkarzynski 2008)Mamluk et al. 1999; Meidan et al. 1999, 2005; Levyet al. 2000). EDN1 <strong>in</strong>hibited P4 secretion, <strong>in</strong> a dosedependent manner via selective EDN1 b<strong>in</strong>d<strong>in</strong>gs sites(EBN A ; Girsh et al. 1996). The expression of membersof the EDN1 system (EDN1, EDN convert<strong>in</strong>g enzymes,and EDN A and EDN B receptors) <strong>in</strong>creases dur<strong>in</strong>g lutealregression (Ohtani et al. 1998; Klipper et al. 2004;Rosiansky-Sultan et al. 2006). Moreover, PGF 2a upregulatesEDN1 and EDN A expression with<strong>in</strong> the CL(Mamluk et al. 1999). Besides EDN1, other vasoactivepeptides [i.e. angiothens<strong>in</strong> II (ANG-II), atrial natriureticpeptide (ANP)] are considered important factors <strong>in</strong>mediat<strong>in</strong>g PGF 2a luteolytic action (Schams and Berisha2004; Berisha and Schams 2005). These vasoactivepeptides decreased blood flow and triggered the luteolyticcascade and consequently <strong>in</strong>hibited P4 secretion(Kobayashi et al. 2002; Shirasuna et al. 2004). However,Flores with colleagues showed that the EDN1 system(EDN1, EDN A and EDN convert<strong>in</strong>g enzymes) exists <strong>in</strong>the bov<strong>in</strong>e CL through the oestrous cycle and PGF 2a<strong>in</strong>creased EDN1 mRNA expression <strong>in</strong> vivo only at 10 hafter treatment (Wright et al. 2001). In fact, Schamset al. (2003) showed that up-regulation of EDN1 andANG-2 occurred ma<strong>in</strong>ly dur<strong>in</strong>g structural CL regression.Therefore, it has been suggested that EDN1 is<strong>in</strong>volved <strong>in</strong> the process of structural CL regression bypromot<strong>in</strong>g leucocyte migration and stimulat<strong>in</strong>g macrophagesto release cytok<strong>in</strong>es [i.e. tumour necrosis factor a(TNF-a), <strong>in</strong>terferon c (IFN-c); Meidan et al. 1999,2005].Cytok<strong>in</strong>esImmune cells <strong>in</strong>filtrat<strong>in</strong>g the bov<strong>in</strong>e CL play a centralrole <strong>in</strong> structural luteolysis of both steroidogenic andendothelial CL cells (Friedman et al. 2000; Pate andLandis Keyes 2001; Pate 2003). The number of leucocytes(i.e. T lymphocytes, macrophages) <strong>in</strong>creased at thetime of structural luteolysis (Penny et al. 1999; Towsonet al. 2002). Shaw and Britt (1995) us<strong>in</strong>g a CL microdialysissystem showed that TNF-a is released dur<strong>in</strong>gspontaneous and PGF 2a -<strong>in</strong>duced luteolysis <strong>in</strong> cows.Then, it was shown that the mRNAs for TNF-a and itsspecific receptors (TNFR type-I) are present <strong>in</strong> thebov<strong>in</strong>e CL dur<strong>in</strong>g luteolysis (Sakumoto et al. 2000;Neuvians et al. 2004). Tumour necrosis factor a <strong>in</strong>comb<strong>in</strong>ation with IFN-c reduced P4 production and<strong>in</strong>duced apoptosis and PGF 2a production by luteal cells<strong>in</strong> vitro (Sakumoto et al. 2000; Petroff et al. 2001;Korzekwa et al. 2006). Specific b<strong>in</strong>d<strong>in</strong>g sites for TNFaare also present <strong>in</strong> endothelial cells derived frombov<strong>in</strong>e CL (Okuda et al. 1999). Furthermore, TNF-a<strong>in</strong>duces EDN1 production by endothelial cells that maylead to structural regression of the CL (Okuda et al.1999; Friedman et al. 2000). Tumour necrosis factor aact<strong>in</strong>g via TNFR type-I <strong>in</strong>duces apoptotic death ofsteroidogenic (Petroff et al. 2001) and endothelial cells(Friedman et al. 2000) of bov<strong>in</strong>e CL. However, some <strong>in</strong>vitro studies <strong>in</strong>dicated that TNF-a <strong>in</strong>duces luteolysisonly <strong>in</strong> comb<strong>in</strong>ation with INFc or other factors (i.e.EDN1; Petroff et al. 2001; Korzekwa et al. 2006).Therefore, we tested whether TNF-a acts as a luteolyticfactor <strong>in</strong> vivo, and whether it changes the lifespan ofbov<strong>in</strong>e CL (Skarzynski et al. 2003a). Lower doses ofTNF-a <strong>in</strong>creased PGF 2a and nitrite ⁄ nitrate (stablemetabolites of NO), decreased P4 level and consequentlyresulted <strong>in</strong> shorten<strong>in</strong>g of the oestrous cycle. Surpris<strong>in</strong>gly,higher doses of TNF-a stimulated the synthesis of P4and PGE 2 and consequently resulted <strong>in</strong> prolongation ofthe oestrous cycle (Skarzynski et al. 2003a). However,<strong>in</strong>hibition of PG synthesis by <strong>in</strong>domethac<strong>in</strong>, a cyclooxygenase(COX; PTGS) <strong>in</strong>hibitor, <strong>in</strong>jected <strong>in</strong>to the aortaabdom<strong>in</strong>alis, blocked the actions of TNF-a <strong>in</strong>dicat<strong>in</strong>gthat TNF-a acts ma<strong>in</strong>ly through mediation by arachidonicacid metabolites (Skarzynski et al. 2007). Inaddition to TNFR, other cytok<strong>in</strong>e membrane receptors,second messengers, <strong>in</strong>clud<strong>in</strong>g calcium ions [Ca 2+ ] i andregulatory prote<strong>in</strong>s are <strong>in</strong>volved <strong>in</strong> apoptosis of steroidogenicand endothelial CL cells (Meidan et al. 1999;Petroff et al. 2001; Taniguchi et al. 2002). Fas ligand, amember of the TNF super family, primarily engages itsreceptors (Fas) to <strong>in</strong>duce apoptosis (Taniguchi et al.2002; Okuda et al. 2004). The expression of Fas mRNAwas <strong>in</strong>creased by IFN-c, and TNF-a augmented thestimulatory action of IFN-c on Fas expression (Taniguchiet al. 2002). Moreover, apoptotic bodies wereobserved <strong>in</strong> luteal cells treated with Fas L <strong>in</strong> thepresence of IFN-c and ⁄ or TNF-a, show<strong>in</strong>g that leucocyte-derivedTNF-a and IFN-c play important roles <strong>in</strong>Fas L-Fas-mediated luteal cell death <strong>in</strong> the bov<strong>in</strong>e CL.Nitric oxideNADPH-d localization [a marker for nitric oxidesynthase (NOS)] and immunosta<strong>in</strong><strong>in</strong>g of both isoformsof NOS [<strong>in</strong>ducible (iNOS) and endothelial (eNOS)] weredetected <strong>in</strong> steroidogenic cells and <strong>in</strong> blood vessels of thebov<strong>in</strong>e CL dur<strong>in</strong>g the entire oestrous cycle with<strong>in</strong>creas<strong>in</strong>g activity from the early to the late lutealphases (Skarzynski et al. 2003b). However, Rosiansky-Sultan et al. (2006) presented the highest level of eNOSand iNOS mRNA and prote<strong>in</strong> expression <strong>in</strong> the earlyÓ 2008 The Authors. Journal compilation Ó 2008 Blackwell Verlag