Reproduction in Domestic Animals
Reproduction in Domestic Animals
Reproduction in Domestic Animals
- No tags were found...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
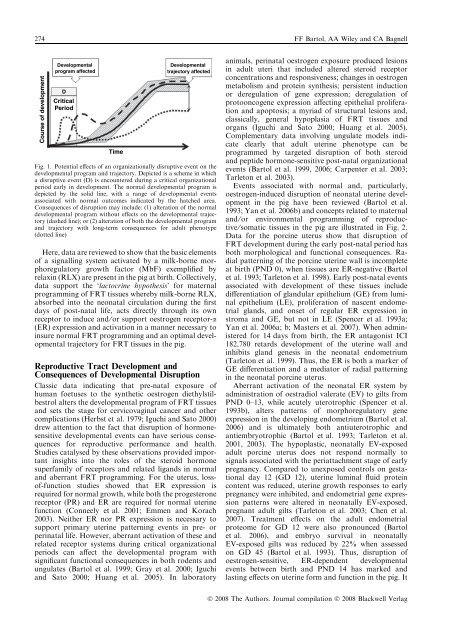
274 FF Bartol, AA Wiley and CA BagnellCourse of developmentDevelopmentalprogram affectedDCriticalPeriodTimeDevelopmentaltrajectory affectedFig. 1. Potential effects of an organizationally disruptive event on thedevelopmental program and trajectory. Depicted is a scheme <strong>in</strong> whicha disruptive event (D) is encountered dur<strong>in</strong>g a critical organizationalperiod early <strong>in</strong> development. The normal developmental program isdepicted by the solid l<strong>in</strong>e, with a range of developmental eventsassociated with normal outcomes <strong>in</strong>dicated by the hatched area.Consequences of disruption may <strong>in</strong>clude: (1) alteration of the normaldevelopmental program without effects on the developmental trajectory(dashed l<strong>in</strong>e); or (2) alteration of both the developmental programand trajectory with long-term consequences for adult phenotype(dotted l<strong>in</strong>e)Here, data are reviewed to show that the basic elementsof a signall<strong>in</strong>g system activated by a milk-borne morphoregulatorygrowth factor (MbF) exemplified byrelax<strong>in</strong> (RLX) are present <strong>in</strong> the pig at birth. Collectively,data support the ‘lactocr<strong>in</strong>e hypothesis’ for maternalprogramm<strong>in</strong>g of FRT tissues whereby milk-borne RLX,absorbed <strong>in</strong>to the neonatal circulation dur<strong>in</strong>g the firstdays of post-natal life, acts directly through its ownreceptor to <strong>in</strong>duce and ⁄ or support oestrogen receptor-a(ER) expression and activation <strong>in</strong> a manner necessary to<strong>in</strong>sure normal FRT programm<strong>in</strong>g and an optimal developmentaltrajectory for FRT tissues <strong>in</strong> the pig.Reproductive Tract Development andConsequences of Developmental DisruptionClassic data <strong>in</strong>dicat<strong>in</strong>g that pre-natal exposure ofhuman foetuses to the synthetic oestrogen diethylstilbestrolalters the developmental program of FRT tissuesand sets the stage for cervicovag<strong>in</strong>al cancer and othercomplications (Herbst et al. 1979; Iguchi and Sato 2000)drew attention to the fact that disruption of hormonesensitivedevelopmental events can have serious consequencesfor reproductive performance and health.Studies catalysed by these observations provided important<strong>in</strong>sights <strong>in</strong>to the roles of the steroid hormonesuperfamily of receptors and related ligands <strong>in</strong> normaland aberrant FRT programm<strong>in</strong>g. For the uterus, lossof-functionstudies showed that ER expression isrequired for normal growth, while both the progesteronereceptor (PR) and ER are required for normal uter<strong>in</strong>efunction (Conneely et al. 2001; Emmen and Korach2003). Neither ER nor PR expression is necessary tosupport primary uter<strong>in</strong>e pattern<strong>in</strong>g events <strong>in</strong> pre- orper<strong>in</strong>atal life. However, aberrant activation of these andrelated receptor systems dur<strong>in</strong>g critical organizationalperiods can affect the developmental program withsignificant functional consequences <strong>in</strong> both rodents andungulates (Bartol et al. 1999; Gray et al. 2000; Iguchiand Sato 2000; Huang et al. 2005). In laboratoryanimals, per<strong>in</strong>atal oestrogen exposure produced lesions<strong>in</strong> adult uteri that <strong>in</strong>cluded altered steroid receptorconcentrations and responsiveness; changes <strong>in</strong> oestrogenmetabolism and prote<strong>in</strong> synthesis; persistent <strong>in</strong>ductionor deregulation of gene expression; deregulation ofprotooncogene expression affect<strong>in</strong>g epithelial proliferationand apoptosis; a myriad of structural lesions and,classically, general hypoplasia of FRT tissues andorgans (Iguchi and Sato 2000; Huang et al. 2005).Complementary data <strong>in</strong>volv<strong>in</strong>g ungulate models <strong>in</strong>dicateclearly that adult uter<strong>in</strong>e phenotype can beprogrammed by targeted disruption of both steroidand peptide hormone-sensitive post-natal organizationalevents (Bartol et al. 1999, 2006; Carpenter et al. 2003;Tarleton et al. 2003).Events associated with normal and, particularly,oestrogen-<strong>in</strong>duced disruption of neonatal uter<strong>in</strong>e development<strong>in</strong> the pig have been reviewed (Bartol et al.1993; Yan et al. 2006b) and concepts related to maternaland ⁄ or environmental programm<strong>in</strong>g of reproductive⁄ somatic tissues <strong>in</strong> the pig are illustrated <strong>in</strong> Fig. 2.Data for the porc<strong>in</strong>e uterus show that disruption ofFRT development dur<strong>in</strong>g the early post-natal period hasboth morphological and functional consequences. Radialpattern<strong>in</strong>g of the porc<strong>in</strong>e uter<strong>in</strong>e wall is <strong>in</strong>completeat birth (PND 0), when tissues are ER-negative (Bartolet al. 1993; Tarleton et al. 1998). Early post-natal eventsassociated with development of these tissues <strong>in</strong>cludedifferentiation of glandular epithelium (GE) from lum<strong>in</strong>alepithelium (LE), proliferation of nascent endometrialglands, and onset of regular ER expression <strong>in</strong>stroma and GE, but not <strong>in</strong> LE (Spencer et al. 1993a;Yan et al. 2006a; b; Masters et al. 2007). When adm<strong>in</strong>isteredfor 14 days from birth, the ER antagonist ICI182,780 retards development of the uter<strong>in</strong>e wall and<strong>in</strong>hibits gland genesis <strong>in</strong> the neonatal endometrium(Tarleton et al. 1999). Thus, the ER is both a marker ofGE differentiation and a mediator of radial pattern<strong>in</strong>g<strong>in</strong> the neonatal porc<strong>in</strong>e uterus.Aberrant activation of the neonatal ER system byadm<strong>in</strong>istration of oestradiol valerate (EV) to gilts fromPND 0–13, while acutely uterotrophic (Spencer et al.1993b), alters patterns of morphoregulatory geneexpression <strong>in</strong> the develop<strong>in</strong>g endometrium (Bartol et al.2006) and is ultimately both antiuterotrophic andantiembryotrophic (Bartol et al. 1993; Tarleton et al.2001, 2003). The hypoplastic, neonatally EV-exposedadult porc<strong>in</strong>e uterus does not respond normally tosignals associated with the periattachment stage of earlypregnancy. Compared to unexposed controls on gestationalday 12 (GD 12), uter<strong>in</strong>e lum<strong>in</strong>al fluid prote<strong>in</strong>content was reduced, uter<strong>in</strong>e growth responses to earlypregnancy were <strong>in</strong>hibited, and endometrial gene expressionpatterns were altered <strong>in</strong> neonatally EV-exposed,pregnant adult gilts (Tarleton et al. 2003; Chen et al.2007). Treatment effects on the adult endometrialproteome for GD 12 were also pronounced (Bartolet al. 2006), and embryo survival <strong>in</strong> neonatallyEV-exposed gilts was reduced by 22% when assessedon GD 45 (Bartol et al. 1993). Thus, disruption ofoestrogen-sensitive, ER-dependent developmentalevents between birth and PND 14 has marked andlast<strong>in</strong>g effects on uter<strong>in</strong>e form and function <strong>in</strong> the pig. ItÓ 2008 The Authors. Journal compilation Ó 2008 Blackwell Verlag