Reproduction in Domestic Animals
Reproduction in Domestic Animals
Reproduction in Domestic Animals
- No tags were found...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
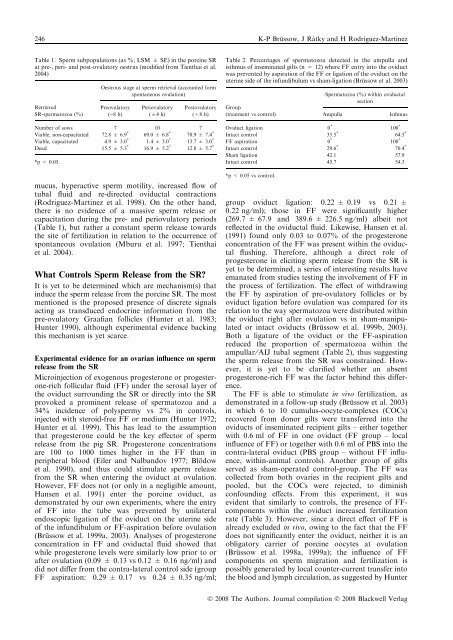
246 K-P Bru¨ ssow, J Rátky and H Rodriguez-Mart<strong>in</strong>ezTable 1. Sperm subpopulations (as %; LSM ± SE) <strong>in</strong> the porc<strong>in</strong>e SRat pre-, peri- and post-ovulatory oestrus (modified from Tienthai et al.2004)RetrievedSR-spermatozoa (%)Oestrous stage at sperm retrieval (accounted formspontaneous ovulation)Preovulatory()8 h)Periovulatory(±4 h)Postovulatory(+8 h)Number of sows 7 10 7Viable, non-capacitated 72.8 ± 6.9 * 69.0 ± 6.8 * 70.9 ± 7.4 *Viable, capacitated 4.9 ± 3.0 * 1.4 ± 3.0 * 13.7 ± 3.0 *Dead 15.5 ± 5.3 * 16.9 ± 5.2 * 12.8 ± 5.7 **p < 0.05.mucus, hyperactive sperm motility, <strong>in</strong>creased flow oftubal fluid and re-directed oviductal contractions(Rodriguez-Mart<strong>in</strong>ez et al. 1998). On the other hand,there is no evidence of a massive sperm release orcapacitation dur<strong>in</strong>g the pre- and periovulatory periods(Table 1), but rather a constant sperm release towardsthe site of fertilization <strong>in</strong> relation to the occurrence ofspontaneous ovulation (Mburu et al. 1997; Tienthaiet al. 2004).What Controls Sperm Release from the SR?It is yet to be determ<strong>in</strong>ed which are mechanism(s) that<strong>in</strong>duce the sperm release from the porc<strong>in</strong>e SR. The mostmentioned is the proposed presence of discrete signalsact<strong>in</strong>g as transduced endocr<strong>in</strong>e <strong>in</strong>formation from thepre-ovulatory Graafian follicles (Hunter et al. 1983;Hunter 1990), although experimental evidence back<strong>in</strong>gthis mechanism is yet scarce.Experimental evidence for an ovarian <strong>in</strong>fluence on spermrelease from the SRMicro<strong>in</strong>jection of exogenous progesterone or progesterone-richfollicular fluid (FF) under the serosal layer ofthe oviduct surround<strong>in</strong>g the SR or directly <strong>in</strong>to the SRprovoked a prom<strong>in</strong>ent release of spermatozoa and a34% <strong>in</strong>cidence of polyspermy vs 2% <strong>in</strong> controls,<strong>in</strong>jected with steroid-free FF or medium (Hunter 1972;Hunter et al. 1999). This has lead to the assumptionthat progesterone could be the key effector of spermrelease from the pig SR. Progesterone concentrationsare 100 to 1000 times higher <strong>in</strong> the FF than <strong>in</strong>peripheral blood (Eiler and Nalbandov 1977; Blo¨ dowet al. 1990), and thus could stimulate sperm releasefrom the SR when enter<strong>in</strong>g the oviduct at ovulation.However, FF does not (or only <strong>in</strong> a negligible amount,Hansen et al. 1991) enter the porc<strong>in</strong>e oviduct, asdemonstrated by our own experiments, where the entryof FF <strong>in</strong>to the tube was prevented by unilateralendoscopic ligation of the oviduct on the uter<strong>in</strong>e sideof the <strong>in</strong>fundibulum or FF-aspiration before ovulation(Bru¨ ssow et al. 1999a, 2003). Analyses of progesteroneconcentration <strong>in</strong> FF and oviductal fluid showed thatwhile progesterone levels were similarly low prior to orafter ovulation (0.09 ± 0.13 vs 0.12 ± 0.16 ng ⁄ ml) anddid not differ from the contra-lateral control side (groupFF aspiration: 0.29 ± 0.17 vs 0.24 ± 0.35 ng ⁄ ml;Table 2. Percentages of spermatozoa detected <strong>in</strong> the ampulla andisthmus of <strong>in</strong>sem<strong>in</strong>ated gilts (n = 12) where FF entry <strong>in</strong>to the oviductwas prevented by aspiration of the FF or ligation of the oviduct on theuter<strong>in</strong>e side of the <strong>in</strong>fundibulum vs sham-ligation (Bru¨ ssow et al. 2003)Group(treatment vs control)Spermatozoa (%) with<strong>in</strong> oviductalsectionAmpullaIsthmusOviduct ligation 0 * 100 *Intact control 35.5 * 64.5 *FF aspiration 0 * 100 *Intact control 29.6 * 70.4 *Sham ligation 42.1 57.9Intact control 45.7 54.3*p < 0.05 vs control.group oviduct ligation: 0.22 ± 0.19 vs 0.21 ±0.22 ng ⁄ ml); those <strong>in</strong> FF were significantly higher(269.7 ± 67.9 and 389.6 ± 226.5 ng ⁄ ml) albeit notreflected <strong>in</strong> the oviductal fluid. Likewise, Hansen et al.(1991) found only 0.03 to 0.07% of the progesteroneconcentration of the FF was present with<strong>in</strong> the oviductalflush<strong>in</strong>g. Therefore, although a direct role ofprogesterone <strong>in</strong> elicit<strong>in</strong>g sperm release from the SR isyet to be determ<strong>in</strong>ed, a series of <strong>in</strong>terest<strong>in</strong>g results haveemanated from studies test<strong>in</strong>g the <strong>in</strong>volvement of FF <strong>in</strong>the process of fertilization. The effect of withdraw<strong>in</strong>gthe FF by aspiration of pre-ovulatory follicles or byoviduct ligation before ovulation was compared for itsrelation to the way spermatozoa were distributed with<strong>in</strong>the oviduct right after ovulation vs <strong>in</strong> sham-manipulatedor <strong>in</strong>tact oviducts (Bru¨ ssow et al. 1999b, 2003).Both a ligature of the oviduct or the FF-aspirationreduced the proportion of spermatozoa with<strong>in</strong> theampullar ⁄ AIJ tubal segment (Table 2), thus suggest<strong>in</strong>gthe sperm release from the SR was constra<strong>in</strong>ed. However,it is yet to be clarified whether an absentprogesterone-rich FF was the factor beh<strong>in</strong>d this difference.The FF is able to stimulate <strong>in</strong> vivo fertilization, asdemonstrated <strong>in</strong> a follow-up study (Bru¨ ssow et al. 2003)<strong>in</strong> which 6 to 10 cumulus-oocyte-complexes (COCs)recovered from donor gilts were transferred <strong>in</strong>to theoviducts of <strong>in</strong>sem<strong>in</strong>ated recipient gilts – either togetherwith 0.6 ml of FF <strong>in</strong> one oviduct (FF group – local<strong>in</strong>fluence of FF) or together with 0.6 ml of PBS <strong>in</strong>to thecontra-lateral oviduct (PBS group – without FF <strong>in</strong>fluence,with<strong>in</strong>-animal controls). Another group of giltsserved as sham-operated control-group. The FF wascollected from both ovaries <strong>in</strong> the recipient gilts andpooled, but the COCs were rejected, to dim<strong>in</strong>ishconfound<strong>in</strong>g effects. From this experiment, it wasevident that similarly to controls, the presence of FFcomponentswith<strong>in</strong> the oviduct <strong>in</strong>creased fertilizationrate (Table 3). However, s<strong>in</strong>ce a direct effect of FF isalready excluded <strong>in</strong> vivo, ow<strong>in</strong>g to the fact that the FFdoes not significantly enter the oviduct, neither it is anobligatory carrier of porc<strong>in</strong>e oocytes at ovulation(Bru¨ ssow et al. 1998a, 1999a); the <strong>in</strong>fluence of FFcomponents on sperm migration and fertilization ispossibly generated by local counter-current transfer <strong>in</strong>tothe blood and lymph circulation, as suggested by HunterÓ 2008 The Authors. Journal compilation Ó 2008 Blackwell Verlag