Reproduction in Domestic Animals
Reproduction in Domestic Animals
Reproduction in Domestic Animals
- No tags were found...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
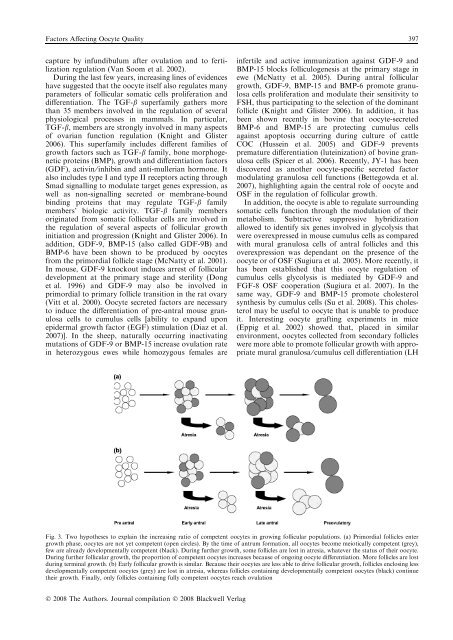
Factors Affect<strong>in</strong>g Oocyte Quality 397capture by <strong>in</strong>fundibulum after ovulation and to fertilizationregulation (Van Soom et al. 2002).Dur<strong>in</strong>g the last few years, <strong>in</strong>creas<strong>in</strong>g l<strong>in</strong>es of evidenceshave suggested that the oocyte itself also regulates manyparameters of follicular somatic cells proliferation anddifferentiation. The TGF-b superfamily gathers morethan 35 members <strong>in</strong>volved <strong>in</strong> the regulation of severalphysiological processes <strong>in</strong> mammals. In particular,TGF-b, members are strongly <strong>in</strong>volved <strong>in</strong> many aspectsof ovarian function regulation (Knight and Glister2006). This superfamily <strong>in</strong>cludes different families ofgrowth factors such as TGF-b family, bone morphogeneticprote<strong>in</strong>s (BMP), growth and differentiation factors(GDF), activ<strong>in</strong> ⁄ <strong>in</strong>hib<strong>in</strong> and anti-mullerian hormone. Italso <strong>in</strong>cludes type I and type II receptors act<strong>in</strong>g throughSmad signall<strong>in</strong>g to modulate target genes expression, aswell as non-signall<strong>in</strong>g secreted or membrane-boundb<strong>in</strong>d<strong>in</strong>g prote<strong>in</strong>s that may regulate TGF-b familymembers’ biologic activity. TGF-b family membersorig<strong>in</strong>ated from somatic follicular cells are <strong>in</strong>volved <strong>in</strong>the regulation of several aspects of follicular growth<strong>in</strong>itiation and progression (Knight and Glister 2006). Inaddition, GDF-9, BMP-15 (also called GDF-9B) andBMP-6 have been shown to be produced by oocytesfrom the primordial follicle stage (McNatty et al. 2001).In mouse, GDF-9 knockout <strong>in</strong>duces arrest of folliculardevelopment at the primary stage and sterility (Donget al. 1996) and GDF-9 may also be <strong>in</strong>volved <strong>in</strong>primordial to primary follicle transition <strong>in</strong> the rat ovary(Vitt et al. 2000). Oocyte secreted factors are necessaryto <strong>in</strong>duce the differentiation of pre-antral mouse granulosacells to cumulus cells [ability to expand uponepidermal growth factor (EGF) stimulation (Diaz et al.2007)]. In the sheep, naturally occurr<strong>in</strong>g <strong>in</strong>activat<strong>in</strong>gmutations of GDF-9 or BMP-15 <strong>in</strong>crease ovulation rate<strong>in</strong> heterozygous ewes while homozygous females are<strong>in</strong>fertile and active immunization aga<strong>in</strong>st GDF-9 andBMP-15 blocks folliculogenesis at the primary stage <strong>in</strong>ewe (McNatty et al. 2005). Dur<strong>in</strong>g antral folliculargrowth, GDF-9, BMP-15 and BMP-6 promote granulosacells proliferation and modulate their sensitivity toFSH, thus participat<strong>in</strong>g to the selection of the dom<strong>in</strong>antfollicle (Knight and Glister 2006). In addition, it hasbeen shown recently <strong>in</strong> bov<strong>in</strong>e that oocyte-secretedBMP-6 and BMP-15 are protect<strong>in</strong>g cumulus cellsaga<strong>in</strong>st apoptosis occurr<strong>in</strong>g dur<strong>in</strong>g culture of cattleCOC (Husse<strong>in</strong> et al. 2005) and GDF-9 preventspremature differentiation (lute<strong>in</strong>ization) of bov<strong>in</strong>e granulosacells (Spicer et al. 2006). Recently, JY-1 has beendiscovered as another oocyte-specific secreted factormodulat<strong>in</strong>g granulosa cell functions (Bettegowda et al.2007), highlight<strong>in</strong>g aga<strong>in</strong> the central role of oocyte andOSF <strong>in</strong> the regulation of follicular growth.In addition, the oocyte is able to regulate surround<strong>in</strong>gsomatic cells function through the modulation of theirmetabolism. Subtractive suppressive hybridizationallowed to identify six genes <strong>in</strong>volved <strong>in</strong> glycolysis thatwere overexpressed <strong>in</strong> mouse cumulus cells as comparedwith mural granulosa cells of antral follicles and thisoverexpression was dependant on the presence of theoocyte or of OSF (Sugiura et al. 2005). More recently, ithas been established that this oocyte regulation ofcumulus cells glycolysis is mediated by GDF-9 andFGF-8 OSF cooperation (Sugiura et al. 2007). In thesame way, GDF-9 and BMP-15 promote cholesterolsynthesis by cumulus cells (Su et al. 2008). This cholesterolmay be useful to oocyte that is unable to produceit. Interest<strong>in</strong>g oocyte graft<strong>in</strong>g experiments <strong>in</strong> mice(Eppig et al. 2002) showed that, placed <strong>in</strong> similarenvironment, oocytes collected from secondary follicleswere more able to promote follicular growth with appropriatemural granulosa ⁄ cumulus cell differentiation (LHFig. 3. Two hypotheses to expla<strong>in</strong> the <strong>in</strong>creas<strong>in</strong>g ratio of competent oocytes <strong>in</strong> grow<strong>in</strong>g follicular populations. (a) Primordial follicles entergrowth phase, oocytes are not yet competent (open circles). By the time of antrum formation, all oocytes become meiotically competent (grey),few are already developmentally competent (black). Dur<strong>in</strong>g further growth, some follicles are lost <strong>in</strong> atresia, whatever the status of their oocyte.Dur<strong>in</strong>g further follicular growth, the proportion of competent oocytes <strong>in</strong>creases because of ongo<strong>in</strong>g oocyte differentiation. More follicles are lostdur<strong>in</strong>g term<strong>in</strong>al growth. (b) Early follicular growth is similar. Because their oocytes are less able to drive follicular growth, follicles enclos<strong>in</strong>g lessdevelopmentally competent oocytes (grey) are lost <strong>in</strong> atresia, whereas follicles conta<strong>in</strong><strong>in</strong>g developmentally competent oocytes (black) cont<strong>in</strong>uetheir growth. F<strong>in</strong>ally, only follicles conta<strong>in</strong><strong>in</strong>g fully competent oocytes reach ovulationÓ 2008 The Authors. Journal compilation Ó 2008 Blackwell Verlag