Reproduction in Domestic Animals
Reproduction in Domestic Animals
Reproduction in Domestic Animals
- No tags were found...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
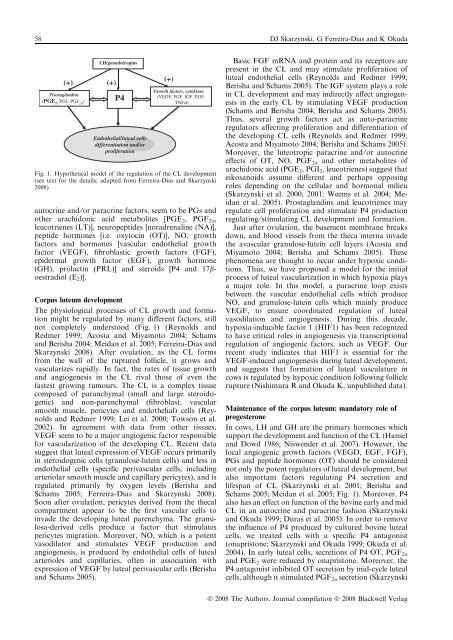
58 DJ Skarzynski, G Ferreira-Dias and K Okuda(+)Prostagland<strong>in</strong>s(PGE 2, PGI 2, PGF 2a )LH/gonadotrop<strong>in</strong>s(+)P4Endothelial/luteal cellsdifferentiation and/orproliferationautocr<strong>in</strong>e and ⁄ or paracr<strong>in</strong>e factors, seem to be PGs andother arachidonic acid metabolites [PGE 2 , PGF 2a ,leucotrienes (LT)], neuropeptides [noradrenal<strong>in</strong>e (NA)],peptide hormones [i.e. oxytoc<strong>in</strong> (OT)], NO, growthfactors and hormones [vascular endothelial growthfactor (VEGF), fibroblastic growth factors (FGF),epidermal growth factor (EGF), growth hormone(GH), prolact<strong>in</strong> (PRL)] and steroids [P4 and 17boestradiol(E 2 )].Corpus luteum developmentThe physiological processes of CL growth and formationmight be regulated by many different factors, stillnot completely understood (Fig. 1) (Reynolds andRedmer 1999; Acosta and Miyamoto 2004; Schamsand Berisha 2004; Meidan et al. 2005; Ferreira-Dias andSkarzynski 2008). After ovulation, as the CL formsfrom the wall of the ruptured follicle, it grows andvascularizes rapidly. In fact, the rates of tissue growthand angiogenesis <strong>in</strong> the CL rival those of even thefastest grow<strong>in</strong>g tumours. The CL is a complex tissuecomposed of paranchymal (small and large steroidogenic)and non-parenchymal (fibroblast, vascularsmooth muscle, pericytes and endothelial) cells (Reynoldsand Redmer 1999; Lei et al. 2000; Towson et al.2002). In agreement with data from other tissues,VEGF seem to be a major angiogenic factor responsiblefor vascularization of the develop<strong>in</strong>g CL. Recent datasuggest that luteal expression of VEGF occurs primarily<strong>in</strong> steroidogenic cells (granulose-lute<strong>in</strong> cells) and less <strong>in</strong>endothelial cells (specific perivascular cells, <strong>in</strong>clud<strong>in</strong>garteriolar smooth muscle and capillary pericytes), and isregulated primarily by oxygen levels (Berisha andSchams 2005; Ferreira-Dias and Skarzynski 2008).Soon after ovulation, pericytes derived from the thecalcompartment appear to be the first vascular cells to<strong>in</strong>vade the develop<strong>in</strong>g luteal parenchyma. The granulosa-derivedcells produce a factor that stimulatespericytes migration. Moreover, NO, which is a potentvasodilator and stimulates VEGF production andangiogenesis, is produced by endothelial cells of lutealarterioles and capillaries, often <strong>in</strong> association withexpression of VEGF by luteal perivascular cells (Berishaand Schams 2005).(+)Growth factors, cytok<strong>in</strong>es(VEGF, FGF, IGF, EGF,TNFa)Fig. 1. Hypothetical model of the regulation of the CL development(see text for the details; adapted from Ferreira-Dias and Skarzynski2008)Basic FGF mRNA and prote<strong>in</strong> and its receptors arepresent <strong>in</strong> the CL and may stimulate proliferation ofluteal endothelial cells (Reynolds and Redmer 1999;Berisha and Schams 2005). The IGF system plays a role<strong>in</strong> CL development and may <strong>in</strong>directly affect angiogenesis<strong>in</strong> the early CL by stimulat<strong>in</strong>g VEGF production(Schams and Berisha 2004; Berisha and Schams 2005).Thus, several growth factors act as auto-paracr<strong>in</strong>eregulators affect<strong>in</strong>g proliferation and differentiation ofthe develop<strong>in</strong>g CL cells (Reynolds and Redmer 1999;Acosta and Miyamoto 2004; Berisha and Schams 2005).Moreover, the luteotropic paracr<strong>in</strong>e and ⁄ or autocr<strong>in</strong>eeffects of OT, NO, PGF 2a and other metabolites ofarachidonic acid (PGE 2 , PGI 2 , leucotrienes) suggest thateikosanoids assume different and perhaps oppos<strong>in</strong>groles depend<strong>in</strong>g on the cellular and hormonal milieu(Skarzynski et al. 2000, 2001; Weems et al. 2004; Meidanet al. 2005). Prostagland<strong>in</strong>s and leucotrienes mayregulate cell proliferation and stimulate P4 productionregulat<strong>in</strong>g ⁄ stimulat<strong>in</strong>g CL development and formation.Just after ovulation, the basement membrane breaksdown, and blood vessels from the theca <strong>in</strong>terna <strong>in</strong>vadethe avascular granulose-lute<strong>in</strong> cell layers (Acosta andMiyamoto 2004; Berisha and Schams 2005). Thesephenomena are thought to occur under hypoxic conditions.Thus, we have proposed a model for the <strong>in</strong>itialprocess of luteal vascularization <strong>in</strong> which hypoxia playsa major role. In this model, a paracr<strong>in</strong>e loop existsbetween the vascular endothelial cells which produceNO, and granulose-lute<strong>in</strong> cells which ma<strong>in</strong>ly produceVEGF, to ensure coord<strong>in</strong>ated regulation of lutealvasodilation and angiogenesis. Dur<strong>in</strong>g this decade,hypoxia-<strong>in</strong>ducible factor 1 (HIF1) has been recognizedto have critical roles <strong>in</strong> angiogenesis via transcriptionalregulation of angiogenic factors, such as VEGF. Ourrecent study <strong>in</strong>dicates that HIF1 is essential for theVEGF-<strong>in</strong>duced angiogenesis dur<strong>in</strong>g luteal development,and suggests that formation of luteal vasculature <strong>in</strong>cows is regulated by hypoxic condition follow<strong>in</strong>g folliclerupture (Nishimura R and Okuda K, unpublished data).Ma<strong>in</strong>tenance of the corpus luteum: mandatory role ofprogesteroneIn cows, LH and GH are the primary hormones whichsupport the development and function of the CL (Hanseland Dowd 1986; Niswender et al. 2007). However, thelocal angiogenic growth factors (VEGD, EGF, FGF),PGs and peptide hormones (OT) should be considerednot only the potent regulators of luteal development, butalso important factors regulat<strong>in</strong>g P4 secretion andlifespan of CL (Skarzynski et al. 2001; Berisha andSchams 2005; Meidan et al. 2005; Fig. 1). Moreover, P4also has an effect on function of the bov<strong>in</strong>e early and midCL <strong>in</strong> an autocr<strong>in</strong>e and paracr<strong>in</strong>e fashion (Skarzynskiand Okuda 1999; Duras et al. 2005). In order to removethe <strong>in</strong>fluence of P4 produced by cultured bov<strong>in</strong>e lutealcells, we treated cells with a specific P4 antagonist(onapristone; Skarzynski and Okuda 1999; Okuda et al.2004). In early luteal cells, secretions of P4 OT, PGF 2aand PGE 2 were reduced by onapristone. Moreover, theP4 antagonist <strong>in</strong>hibited OT secretion by mid-cycle lutealcells, although it stimulated PGF 2a secretion (SkarzynskiÓ 2008 The Authors. Journal compilation Ó 2008 Blackwell Verlag