Reproduction in Domestic Animals
Reproduction in Domestic Animals
Reproduction in Domestic Animals
- No tags were found...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
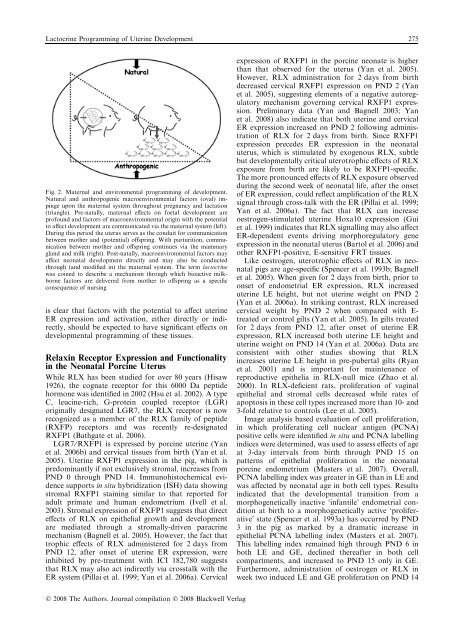
Lactocr<strong>in</strong>e Programm<strong>in</strong>g of Uter<strong>in</strong>e Development 275Fig. 2. Maternal and environmental programm<strong>in</strong>g of development.Natural and anthropogenic macroenvironmental factors (oval) imp<strong>in</strong>geupon the maternal system throughout pregnancy and lactation(triangle). Pre-natally, maternal effects on foetal development areprofound and factors of macroenvironmental orig<strong>in</strong> with the potentialto affect development are communicated via the maternal system (left).Dur<strong>in</strong>g this period the uterus serves as the conduit for communicationbetween mother and (potential) offspr<strong>in</strong>g. With parturition, communicationbetween mother and offspr<strong>in</strong>g cont<strong>in</strong>ues via the mammarygland and milk (right). Post-natally, macroenvironmental factors mayaffect neonatal development directly and may also be conductedthrough (and modified <strong>in</strong>) the maternal system. The term lactocr<strong>in</strong>ewas co<strong>in</strong>ed to describe a mechanism through which bioactive milkbornefactors are delivered from mother to offspr<strong>in</strong>g as a specificconsequence of nurs<strong>in</strong>gis clear that factors with the potential to affect uter<strong>in</strong>eER expression and activation, either directly or <strong>in</strong>directly,should be expected to have significant effects ondevelopmental programm<strong>in</strong>g of these tissues.Relax<strong>in</strong> Receptor Expression and Functionality<strong>in</strong> the Neonatal Porc<strong>in</strong>e UterusWhile RLX has been studied for over 80 years (Hisaw1926), the cognate receptor for this 6000 Da peptidehormone was identified <strong>in</strong> 2002 (Hsu et al. 2002). A typeC, leuc<strong>in</strong>e-rich, G-prote<strong>in</strong> coupled receptor (LGR)orig<strong>in</strong>ally designated LGR7, the RLX receptor is nowrecognized as a member of the RLX family of peptide(RXFP) receptors and was recently re-designatedRXFP1 (Bathgate et al. 2006).LGR7 ⁄ RXFP1 is expressed by porc<strong>in</strong>e uter<strong>in</strong>e (Yanet al. 2006b) and cervical tissues from birth (Yan et al.2005). Uter<strong>in</strong>e RXFP1 expression <strong>in</strong> the pig, which ispredom<strong>in</strong>antly if not exclusively stromal, <strong>in</strong>creases fromPND 0 through PND 14. Immunohistochemical evidencesupports <strong>in</strong> situ hybridization (ISH) data show<strong>in</strong>gstromal RXFP1 sta<strong>in</strong><strong>in</strong>g similar to that reported foradult primate and human endometrium (Ivell et al.2003). Stromal expression of RXFP1 suggests that directeffects of RLX on epithelial growth and developmentare mediated through a stromally-driven paracr<strong>in</strong>emechanism (Bagnell et al. 2005). However, the fact thattrophic effects of RLX adm<strong>in</strong>istered for 2 days fromPND 12, after onset of uter<strong>in</strong>e ER expression, were<strong>in</strong>hibited by pre-treatment with ICI 182,780 suggeststhat RLX may also act <strong>in</strong>directly via crosstalk with theER system (Pillai et al. 1999; Yan et al. 2006a). Cervicalexpression of RXFP1 <strong>in</strong> the porc<strong>in</strong>e neonate is higherthan that observed for the uterus (Yan et al. 2005).However, RLX adm<strong>in</strong>istration for 2 days from birthdecreased cervical RXFP1 expression on PND 2 (Yanet al. 2005), suggest<strong>in</strong>g elements of a negative autoregulatorymechanism govern<strong>in</strong>g cervical RXFP1 expression.Prelim<strong>in</strong>ary data (Yan and Bagnell 2003; Yanet al. 2008) also <strong>in</strong>dicate that both uter<strong>in</strong>e and cervicalER expression <strong>in</strong>creased on PND 2 follow<strong>in</strong>g adm<strong>in</strong>istrationof RLX for 2 days from birth. S<strong>in</strong>ce RXFP1expression precedes ER expression <strong>in</strong> the neonataluterus, which is stimulated by exogenous RLX, subtlebut developmentally critical uterotrophic effects of RLXexposure from birth are likely to be RXFP1-specific.The more pronounced effects of RLX exposure observeddur<strong>in</strong>g the second week of neonatal life, after the onsetof ER expression, could reflect amplification of the RLXsignal through cross-talk with the ER (Pillai et al. 1999;Yan et al. 2006a). The fact that RLX can <strong>in</strong>creaseoestrogen-stimulated uter<strong>in</strong>e Hoxa10 expression (Guiet al. 1999) <strong>in</strong>dicates that RLX signall<strong>in</strong>g may also affectER-dependent events driv<strong>in</strong>g morphoregulatory geneexpression <strong>in</strong> the neonatal uterus (Bartol et al. 2006) andother RXFP1-positive, E-sensitive FRT tissues.Like oestrogen, uterotrophic effects of RLX <strong>in</strong> neonatalpigs are age-specific (Spencer et al. 1993b; Bagnellet al. 2005). When given for 2 days from birth, prior toonset of endometrial ER expression, RLX <strong>in</strong>creaseduter<strong>in</strong>e LE height, but not uter<strong>in</strong>e weight on PND 2(Yan et al. 2006a). In strik<strong>in</strong>g contrast, RLX <strong>in</strong>creasedcervical weight by PND 2 when compared with E-treated or control gilts (Yan et al. 2005). In gilts treatedfor 2 days from PND 12, after onset of uter<strong>in</strong>e ERexpression, RLX <strong>in</strong>creased both uter<strong>in</strong>e LE height anduter<strong>in</strong>e weight on PND 14 (Yan et al. 2006a). Data areconsistent with other studies show<strong>in</strong>g that RLX<strong>in</strong>creases uter<strong>in</strong>e LE height <strong>in</strong> pre-pubertal gilts (Ryanet al. 2001) and is important for ma<strong>in</strong>tenance ofreproductive epithelia <strong>in</strong> RLX-null mice (Zhao et al.2000). In RLX-deficient rats, proliferation of vag<strong>in</strong>alepithelial and stromal cells decreased while rates ofapoptosis <strong>in</strong> these cell types <strong>in</strong>creased more than 10- and3-fold relative to controls (Lee et al. 2005).Image analysis based evaluation of cell proliferation,<strong>in</strong> which proliferat<strong>in</strong>g cell nuclear antigen (PCNA)positive cells were identified <strong>in</strong> situ and PCNA labell<strong>in</strong>g<strong>in</strong>dices were determ<strong>in</strong>ed, was used to assess effects of ageat 3-day <strong>in</strong>tervals from birth through PND 15 onpatterns of epithelial proliferation <strong>in</strong> the neonatalporc<strong>in</strong>e endometrium (Masters et al. 2007). Overall,PCNA labell<strong>in</strong>g <strong>in</strong>dex was greater <strong>in</strong> GE than <strong>in</strong> LE andwas affected by neonatal age <strong>in</strong> both cell types. Results<strong>in</strong>dicated that the developmental transition from amorphogenetically <strong>in</strong>active ‘<strong>in</strong>fantile’ endometrial conditionat birth to a morphogenetically active ‘proliferative’state (Spencer et al. 1993a) has occurred by PND3 <strong>in</strong> the pig as marked by a dramatic <strong>in</strong>crease <strong>in</strong>epithelial PCNA labell<strong>in</strong>g <strong>in</strong>dex (Masters et al. 2007).This labell<strong>in</strong>g <strong>in</strong>dex rema<strong>in</strong>ed high through PND 6 <strong>in</strong>both LE and GE, decl<strong>in</strong>ed thereafter <strong>in</strong> both cellcompartments, and <strong>in</strong>creased to PND 15 only <strong>in</strong> GE.Furthermore, adm<strong>in</strong>istration of oestrogen or RLX <strong>in</strong>week two <strong>in</strong>duced LE and GE proliferation on PND 14Ó 2008 The Authors. Journal compilation Ó 2008 Blackwell Verlag