- Page 1 and 2:
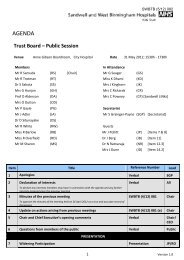
SWBTB (2/10) 026 AGENDA Trust Board
- Page 3 and 4:
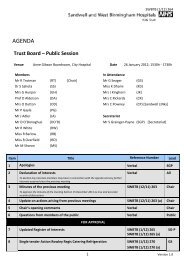
SWBTB (2/10) 026 18 Any other busin
- Page 5 and 6:
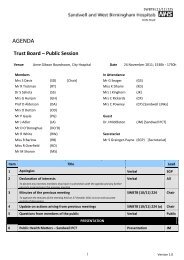
MINUTES approved as true and accura
- Page 7 and 8:
MINUTES expectation that there woul
- Page 9 and 10:
MINUTES however the structure would
- Page 11 and 12:
MINUTES which was reported to be un
- Page 13 and 14:
MINUTES Mr Cash asked which communi
- Page 15 and 16:
MINUTES Mr Seager presented an upda
- Page 17 and 18:
SWBTB (2/10) 025 (a) Next Meeting:
- Page 19 and 20:
SWBTB (2/10) 035 TRUST BOARD DOCUME
- Page 21 and 22:
SWBTB (2/10) 035 PREVIOUS CONSIDERA
- Page 23 and 24:
SWBTB (2/10) 035 (a) Contents Page
- Page 25 and 26:
SWBTB (2/10) 035 (a) 2. Methodology
- Page 27 and 28:
SWBTB (2/10) 035 (a) 2.3 Reach 780
- Page 29 and 30:
SWBTB (2/10) 035 (a) 7
- Page 31 and 32:
SWBTB (2/10) 035 (a) 3. Option Pref
- Page 33 and 34:
SWBTB (2/10) 035 (a) Table 3 Comple
- Page 35 and 36:
SWBTB (2/10) 035 (a) • A number o
- Page 37 and 38:
SWBTB (2/10) 035 (a) • Satisfied
- Page 39 and 40:
SWBTB (2/10) 035 (a) • Bad experi
- Page 41 and 42:
SWBTB (2/10) 035 (a) Table 5 Focus
- Page 43 and 44:
SWBTB (2/10) 035 (a) Some women kne
- Page 45 and 46:
SWBTB (2/10) 035 (a) • Reputation
- Page 47 and 48:
SWBTB (2/10) 035 (a) Young people p
- Page 49 and 50:
SWBTB (2/10) 035 (a) Table 7 Showin
- Page 51 and 52:
SWBTB (2/10) 035 (a) home birth wou
- Page 53 and 54:
SWBTB (2/10) 035 (a) Black Caribbea
- Page 55 and 56:
SWBTB (2/10) 035 (a) White and Blac
- Page 57 and 58:
SWBTB (2/10) 035 (a) • Labour Par
- Page 59 and 60:
SWBTB (2/10) 035 (a) Role Number of
- Page 61 and 62:
SWBTB (2/10) 035 (a) Appendix 1 Foc
- Page 63 and 64:
SWBTB (2/10) 035 (a) Appendix 2 Pro
- Page 65 and 66:
SWBTB (2/10) 035 (a) Postcode Area
- Page 67 and 68:
SWBTB (2/10) 035 (a) Chart 2: Women
- Page 69 and 70:
SWBTB (2/10) 035 (a) Chart 6: Women
- Page 71 and 72:
SWBTB (2/10) 035 (a) Chart 10: Wome
- Page 73 and 74:
SWBTB (2/10) 035 (a) Appendix 5 Com
- Page 75 and 76:
SWBTB (2/10) 035 (a) Appendix 7 Bir
- Page 77 and 78:
SWBTB (2/10) 045 TRUST BOARD DOCUME
- Page 79 and 80:
SWBTB (2/10) 045 IMPACT ASSESSMENT
- Page 81 and 82:
SWBTB (2/10) 045 (a) MATERNITY SERV
- Page 83 and 84:
SWBTB (2/10) 045 (a) All consultant
- Page 85 and 86:
SWBTB (2/10) 045 (a) • APPROVE th
- Page 87 and 88:
SWBTB (2/10) 045 (a) strategy the S
- Page 89 and 90:
SWBTB (2/10) 045 (a) These efforts
- Page 91 and 92:
SWBTB (2/10) 045 (a) risk women in
- Page 93 and 94:
SWBTB (2/10) 045 (a) • Shorter la
- Page 95 and 96:
SWBTB (2/10) 045 (a) Benefit Descri
- Page 97 and 98:
SWBTB (2/10) 045 (a) From the compl
- Page 99 and 100:
SWBTB (2/10) 045 (a) Concerns about
- Page 101 and 102:
SWBTB (2/10) 045 (a) Stages 5 - 9:
- Page 103 and 104:
SWBTB (2/10) 045 (a) The scheme dur
- Page 105 and 106:
SWBTB (2/10) 045 (a) • The stand
- Page 107 and 108:
SWBTB (2/10) 045 (a) 10. INVESTMENT
- Page 109 and 110:
SWBTB (2/10) 045 (a) 11.2 Activity
- Page 111 and 112:
SWBTB (2/10) 045 (a) 13. CASHFLOW P
- Page 113 and 114:
SWBTB (2/10) 045 (a) 15. JOINT HEAL
- Page 115 and 116:
SWBTB (2/10) 045 (a) Sandwell PCT w
- Page 117 and 118:
SWBTB (2/10) 045 (a) APPENDIX 7 DOC
- Page 119 and 120:
SWBTB (2/10) 045 (b) Outpatients Fi
- Page 121 and 122:
SWBTB (2/10) 045 (b) Sandwell & Wes
- Page 123 and 124: Sandwell & West Birmingham Hospital
- Page 125 and 126: Risk Register APPENDIX 7 Version 1
- Page 127 and 128: Risk Register APPENDIX 7 Version 1
- Page 129 and 130: Risk Register Impact Narrative Poss
- Page 131 and 132: SWBTB (2/10) 036 ALIGNMENT TO OBJEC
- Page 133 and 134: POLICY PROFILE 2 SWBTB (2/10) 036 (
- Page 135 and 136: Contents page SWBTB (2/10) 036 (a)
- Page 137 and 138: Infection Control Surveillance Nurs
- Page 139 and 140: 5.4.5 Infection Control Surveillanc
- Page 141 and 142: SWBTB (2/10) 036 (a) i) To circulat
- Page 143 and 144: SWBTB (2/10) 036 (a) 6.3.2. Lines o
- Page 145 and 146: 12.0 Monitoring Effectiveness SWBTB
- Page 147 and 148: SWBTB (2/10) 036 (b) Step 2 - Gathe
- Page 149 and 150: SWBTB (2/10) 036 (c) Appendix 5 POL
- Page 151 and 152: SWBTB (2/10) 036 (c) Identify which
- Page 153 and 154: SWBTB (2/10) 037 TRUST BOARD DOCUME
- Page 155 and 156: SWBTB (2/10) 037 (a) 1 Introduction
- Page 157 and 158: SWBTB (2/10) 037 (a) including the
- Page 159 and 160: SWBTB (2/10) 037 (a) • Has the ab
- Page 161 and 162: SWBTB (2/10) 037 (a) either through
- Page 163 and 164: SWBTB (2/10) 037 (a) If a proposed
- Page 165 and 166: SWBTB (2/10) 037 (a) The provision
- Page 167 and 168: SWBTB (2/10) 037 (a) Staff who are
- Page 169 and 170: • Does this decision need to be m
- Page 171 and 172: SWBTB (2/10) 037 (a) and hence impr
- Page 173: SWBTB (2/10) 037 (a) The consultant
- Page 177 and 178: SWBTB (2/10) 037 (a) • Procedures
- Page 179 and 180: SWBTB (2/10) 037 (b) 1 Appendix A 1
- Page 181 and 182: Appendix A: Consent Form 1 - Right
- Page 183 and 184: SWBTB (2/10) 037 (b) You should alw
- Page 185 and 186: Appendix A: Consent Form 2 - Right
- Page 187 and 188: SWBTB (2/10) 037 (b) appointed guar
- Page 189 and 190: SWBTB (2/10) 037 (b) Form 3 guidanc
- Page 191 and 192: Appendix A: Consent Form 4 - Left h
- Page 193 and 194: SWBTB (2/10) 037 (b) Appendix A: Co
- Page 195 and 196: SWBTB (2/10) 037 (b) Second opinion
- Page 197 and 198: SWBTB (2/10) 037 (b) Appendix C San
- Page 199 and 200: SWBTB (2/10) 037 (b) The young pers
- Page 201 and 202: SWBTB (2/10) 037 (b) Appendix D Fun
- Page 203 and 204: SWBTB (2/10) 037 (b) Appendix E How
- Page 205 and 206: SWBTB (2/10) 037 (b) Appendix G THE
- Page 207 and 208: SWBTB (2/10) 037 (b) Appendix G3 Tr
- Page 209 and 210: SWBTB (2/10) 037 (d) Sandwell and W
- Page 211 and 212: SWBTB (2/10) 037 (d) Introduction T
- Page 213 and 214: SWBTB (2/10) 037 (d) What are the m
- Page 215 and 216: SWBTB (2/10) 037 (d) How do I begin
- Page 217 and 218: Frequently asked Questions SWBTB (2
- Page 219 and 220: SAPG OCT 09 - 11 - SWBTB (2/10) 037
- Page 221 and 222: SWBTB (2/10) 037 (d) Q5) Who was in
- Page 223 and 224: SWBTB (2/10) 037 (d) Appendix B San
- Page 225 and 226:
SWBTB (2/10) 037 (d) 4d The EIA rev
- Page 227 and 228:
SWBTB (2/10) 037 (d) Date: Contact
- Page 230 and 231:
SWBTB (2/10) 037 (e) KEY TASKS ISSU
- Page 232 and 233:
KEY TASKS ISSUES IDENTIFIED ACTION
- Page 234 and 235:
SWBTB (2/10) 029 ALIGNMENT TO OBJEC
- Page 236 and 237:
SWBTB (2/10) 038 ALIGNMENT TO TRUST
- Page 238 and 239:
SWBTB (2/10) 038 (a) From 2010/11 M
- Page 240 and 241:
SWBTB (2/10) 038 (a) Median rate 2.
- Page 242 and 243:
SWBTB (2/10) 038 (a) Audit and Trai
- Page 244 and 245:
SWBTB (2/10) 046 ALIGNMENT TO OBJEC
- Page 246 and 247:
SWBTB (2/10) 046 (a) Compliance Cri
- Page 248 and 249:
3 Provide suitable and sufficient i
- Page 250 and 251:
8 Have and adhere to appropriate po
- Page 252 and 253:
SWBTB (2/10) 027 ALIGNMENT TO OBJEC
- Page 254 and 255:
SWBTB (2/10) 027 (a) PEAT Expendit
- Page 256 and 257:
SWBTB (2/10) 027 (a) A study was co
- Page 258 and 259:
SWBTB (2/10) 039 ALIGNMENT TO OBJEC
- Page 260 and 261:
SWBTB (2/10) 039 (a) Theme Triggers
- Page 262 and 263:
SWBTB (2/10) 029 TRUST BOARD DOCUME
- Page 264 and 265:
SWBTB (2/10) 029 (a) INTRODUCTION S
- Page 266 and 267:
In terms of affordability the follo
- Page 268 and 269:
SMOCS Sandwell PEC HoBt PEC Childre
- Page 270 and 271:
As reported last month, the date fo
- Page 272 and 273:
Les Williams Programme Director 201
- Page 274 and 275:
Respiratory The Project Lead post h
- Page 276 and 277:
SWBTB (2/10) 029 (b) Sandwell and t
- Page 278 and 279:
SWBTB (2/10) 028 ALIGNMENT TO OBJEC
- Page 280 and 281:
With the revised activity / afforda
- Page 282 and 283:
SWBTB (2/10) 041 ALIGNMENT TO OBJEC
- Page 284 and 285:
SWBTB (2/10) 041 (a) • Release 4
- Page 286 and 287:
SWBTB (2/10) 041 (a) • Rotawatch
- Page 288 and 289:
SWBTB (2/10) 030 TRUST BOARD DOCUME
- Page 290 and 291:
SWBGB NEW REF SWBTB (2/10) 030 (a)
- Page 292 and 293:
SWBGB NEW REF SWBTB (2/10) 030 (a)
- Page 294 and 295:
SWBGB NEW REF SWBTB (2/10) 030 (a)
- Page 296 and 297:
SWBGB NEW REF SWBTB (2/10) 030 (a)
- Page 298 and 299:
SWBGB NEW REF SWBTB (2/10) 030 (a)
- Page 300 and 301:
SWBTB (2/10) 031 TRUST BOARD DOCUME
- Page 302 and 303:
SWBTB (2/10) 031 (a) Financial Perf
- Page 304 and 305:
SWBTB (2/10) 031 (a) Financial Perf
- Page 306 and 307:
SWBTB (2/10) 031 (a) Financial Perf
- Page 308 and 309:
SWBTB (2/10) 031 (a) Financial Perf
- Page 310 and 311:
SWBTB (2/10) 031 (a) Financial Perf
- Page 312 and 313:
SWBTB (2/10) 044 TRUST BOARD DOCUME
- Page 314 and 315:
SANDWELL AND WEST BIRMINGHAM HOSPIT
- Page 316 and 317:
Exec Lead RK R0 Readmission Rates I
- Page 318 and 319:
Exec Lead RK RK YTD 09/10 No. 6388
- Page 320 and 321:
SWBTB (2/10) 042 TRUST BOARD DOCUME
- Page 322 and 323:
SWBTB (2/10) 042 (a) SANDWELL AND W
- Page 324 and 325:
SWBTB (2/10) 043 DOCUMENT TITLE: SP
- Page 326 and 327:
SWBTB (2/10) 043 (a) DEVELOPING THE
- Page 328 and 329:
SWBTB (2/10) 043 (a) • Merge the
- Page 330 and 331:
SWBFC (1/10) 010 Finance and Perfor
- Page 332 and 333:
SWBFC (1/10) 010 this be adopted as
- Page 334 and 335:
SWBFC (1/10) 010 The Committee was
- Page 336:
SWBFC (1/10) 010 8.2 Actions and de