Microseismic Monitoring and Geomechanical Modelling of CO2 - bris
Microseismic Monitoring and Geomechanical Modelling of CO2 - bris
Microseismic Monitoring and Geomechanical Modelling of CO2 - bris
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
1.2. CCS OVERVIEW<br />
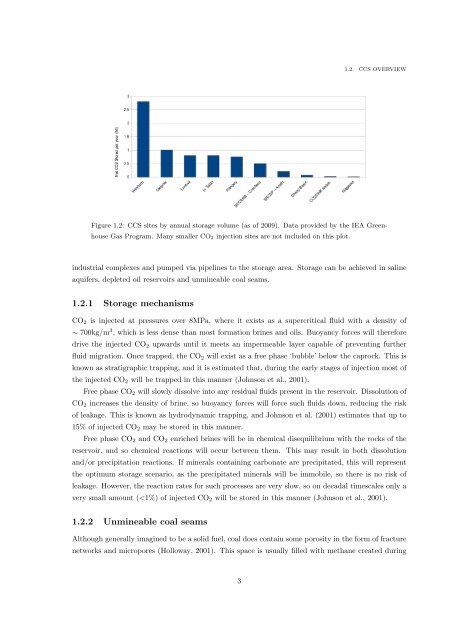
Figure 1.2: CCS sites by annual storage volume (as <strong>of</strong> 2009). Data provided by the IEA Greenhouse<br />
Gas Program. Many smaller CO 2 injection sites are not included on this plot.<br />
industrial complexes <strong>and</strong> pumped via pipelines to the storage area. Storage can be achieved in saline<br />
aquifers, depleted oil reservoirs <strong>and</strong> unmineable coal seams.<br />
1.2.1 Storage mechanisms<br />
CO 2 is injected at pressures over 8MPa, where it exists as a supercritical fluid with a density <strong>of</strong><br />
∼ 700kg/m 3 , which is less dense than most formation brines <strong>and</strong> oils. Buoyancy forces will therefore<br />
drive the injected CO 2 upwards until it meets an impermeable layer capable <strong>of</strong> preventing further<br />
fluid migration. Once trapped, the CO 2 will exist as a free phase ‘bubble’ below the caprock. This is<br />
known as stratigraphic trapping, <strong>and</strong> it is estimated that, during the early stages <strong>of</strong> injection most <strong>of</strong><br />
the injected CO 2 will be trapped in this manner (Johnson et al., 2001).<br />
Free phase CO 2 will slowly dissolve into any residual fluids present in the reservoir. Dissolution <strong>of</strong><br />
CO 2 increases the density <strong>of</strong> brine, so buoyancy forces will force such fluids down, reducing the risk<br />
<strong>of</strong> leakage. This is known as hydrodynamic trapping, <strong>and</strong> Johnson et al. (2001) estimates that up to<br />
15% <strong>of</strong> injected CO 2 may be stored in this manner.<br />
Free phase CO 2 <strong>and</strong> CO 2 enriched brines will be in chemical disequilibrium with the rocks <strong>of</strong> the<br />
reservoir, <strong>and</strong> so chemical reactions will occur between them. This may result in both dissolution<br />
<strong>and</strong>/or precipitation reactions. If minerals containing carbonate are precipitated, this will represent<br />
the optimum storage scenario, as the precipitated minerals will be immobile, so there is no risk <strong>of</strong><br />
leakage. However, the reaction rates for such processes are very slow, so on decadal timescales only a<br />
very small amount (