40. Krege S, Souchon R, Schmoll HJ: Interdisciplinary consensus on diagnosis and treatment of testiculargerm cell tumours: result of an updated conference on evidence-based medicine. Eur Urol, 40: 373-391, 2001.41. Domont J, Laplanche A, de Crevoisier R, Theodore, P. Wibault, K. Fizazi: A risk-adapted strategy ofradiotherapy and cisplatin-based chemotherapy in stage II seminoma: results of a 20-year experience.Proc Am Soc Clin Oncol, 2005; 4571 (abstr)42. Albers P, Siener R, Kliesch S, Weissbach L, Krege S, Sparwasser C, Schulze H, Heidenreich A, de RieseW, Loy V, Bierhoff E, W<strong>it</strong>tekind C, Fimmers R, Hartmann M: Risk factors for relapse in clinical stagenonseminomatous testicular germ cell tumors: results of the German Testicular Cancer Study Grouptrial. J Clin Oncol, 21: 1505-1512, 2003.43. Madej G, Pawinski A: Risk-related adjuvant chemotherapy for stage I non-seminoma of the testis. ClinOncol, 3: 270-272, 1991.44. Freedman LS, Parkinson MC, Jones WG, Oliver RT, Peckham MJ, Read G, Newlands ES, Williams CJ:Histopathology in the prediction of relapse of patients w<strong>it</strong>h stage I testicular teratoma treated byorchiectomy alone. Lancet, 2: 294-298, 1987.45. Read G, Stenning SP, Cullen MH, Parkinson MC, Horwich A, Kaye SB, Cook PA: Medical ResearchCouncil prospective study of surveillance for stage I testicular teratoma. Medical Research CouncilTesticular Tumors Working Party. J Clin Oncol, 10: 1762, 1992.46. Nicolai N, Miceli R, Artusi R, Piva L, Pizzocaro G: A simple model for predicting nodal metastasis inpatients w<strong>it</strong>h clinical stage I nonseminomatous germ cell testicular tumors undergoing retroper<strong>it</strong>oneallymph node dissection. J Urol, 171: 172-176, 2004.47. Pont J, Holtl W, Kosak D, Machacek E, Kienzer H, Julcher H, Honetz N: Risk-adapted treatment choicein stage I nonseminomatous testicular germ cell cancer by regarding vascular invasion in the primarytumor: a prospective trial. J Clin Oncol, 8: 16-19, 1990.48. Cullen MH, Stenning SP, Parkinson MC, Fossa SD, Kaye SB, Horwich AH, Harland SJ, Williams MV,Jakes R: Short-course adjuvant chemotherapy in high-risk stage I nonseminomatous germ cell tumorsof the testis: a Medical Research Council report. J Clin Oncol, 14: 1106, 1996.49. Nicolai N, Pizzocaro G: A surveillance study of clinical stage I nonseminomatous germ cell tumors ofthe testis: 10-year follow-up. J Urol, 154: 1045, 1995.50. Langstroer P, Rosen MA, Griebling TL, Thrasher B: Ejaculatory function in stage T1 nonseminomatousgerm cell tumors: retroper<strong>it</strong>oneal lymph node dissection versus surveillance-a decision analysis. J Urol,168: 1396-1401, 2002.51. Donohue JP, Thornhill JA, Foster RS, Rowland RG, Bihrle R: Retroper<strong>it</strong>oneal lymphadenectomy for clinicalstage A testis cancer (1965 to 1989): modifications of technique and impact on ejaculation. JUrol, 149: 237, 1993.52. Travis LB, Fosså SD, Schonfeld SJ, McMaster ML, Lynch CF, Storm H, Hall P, Holowaty E, Andersen A,Pukkala E, Andersson M, Kaijser M, Gospodarowicz M, Joensuu T, Cohen RJ, Boice JD Jr, Dores GM,Gilbert ES: Second Cancers Among 40,576 Testicular Cancer Patients: Focus on Long-term Survivors.J Natl Cancer Inst, 97: 1354-1365, 2005.53. Culine S, Theodore C, Terrier-Lacombe MJ, Droz JP: Primary chemotherapy in patients w<strong>it</strong>h nonseminomatousgerm cell tumors of the testis and biological disease only after orchiectomy. J Urol, 155:1296-1298, 1996.54. Davis BE, Herr HW, Fair WR, Bosl GJ: The management of patients w<strong>it</strong>h nonseminomatous germ celltumors of the testis w<strong>it</strong>h serologic disease only after orchiectomy. J Urol, 152: 111-113, 1994.55. Weissbach L, Bussar-Maatz R, Flechtner H, Pichlmeier U, Hartmann M, Keller L: RPLND or primary chemotherapyin clinical stage IIA/B nonseminomatous germ cell tumors? Results of a prospective multicentertrial including qual<strong>it</strong>y of life assessment. Eur Urol, 37: 582-594, 2000.56. Neyer M, Peschel R, Akkad T, Springer-Stöhr B, Berger A, Bartsch G, Steiner H: Long-term results oflaparoscopic retroper<strong>it</strong>oneal lymph-node dissection for clinical stage I non seminomatous germ-celltesticular cancer. J Endourol, 21: 180-183, 2007.57. Pizzocaro G, Monfardini S: No adjuvant chemotherapy in selected patients w<strong>it</strong>h pathologic stage IInonseminomatous germ cell tumors of the testis. J Urol, 131: 677-680, 1984.58. Williams SD, Stablein DM, Einhorn LH, Muggia FM, Weiss RB, Donohue JP, Paulson DF, Brunner KW,Jacobs EM, Spaulding JT: Immediate adjuvant chemotherapy versus observation w<strong>it</strong>h treatment atrelapse in pathological stage II testicular cancer patients. N Engl J Med, 317: 1433-1438, 1987.214
59. Weissbach L, Hartlapp JH: Adjuvant chemotherapy of metastatic stage II nonseminomatous testis tumor. JUrol, 146: 1295-1298, 1991.60. Kondagunta GV, Sheinfeld J, Mazumdar M, Mariani TV, Bajorin D, Bacik J, Bosl GJ, Motzer RJ: Relapse-freeand overall survival in patients w<strong>it</strong>h pathologic stage II nonseminomatous germ cell cancer treated w<strong>it</strong>hetoposide and cisplatin adjuvant chemotherapy. J Clin Oncol, 22: 464-467, 2004.61. Gregory C, Peckham MJ: Results of radiotherapy for stage II testicular seminoma. Radiother Oncol, 6: 285-292, 1986.62. Willan BD, McGowan DG: Seminoma of the testis: a 22-year experience w<strong>it</strong>h radiation therapy. Int J RadiatOncol Biol Phys, 11: 1769-1775, 1985.63. de W<strong>it</strong> R, Roberts JT, Wilkinson PM, de Mulder PH, Mead GM, Fossa SD, Cook P, de Prijck L, Stenning S,Collette L: Equivalence of three or four cycles of bleomycin, etoposide, and cisplatin chemotherapy and ofa 3- or 5-day schedule in good-prognosis germ cell cancer: a randomized study of the EuropeanOrganization for Research and Treatment of Cancer Gen<strong>it</strong>ourinary Tract Cancer Cooperative Group and theMedical Research Council. J Clin Oncol, 19: 1629-1640, 2001.64. Fossa SD, de W<strong>it</strong> R, Roberts JT, Wilkinson PM, de Mulder PH, Mead GM, Cook P, de Prijck L, Stenning S,Aaronson NK, Bottomley A, Collette L: Qual<strong>it</strong>y of life in good prognosis patients w<strong>it</strong>h metastatic germ cellcancer: a prospective study of the European Organization for Research and Treatment of CancerGen<strong>it</strong>ourinary Group/Medical Research Council Testicular Cancer Study Group (30941/TE20). J ClinOncol, 21: 1107-1118, 2003.65. Sm<strong>it</strong>h TJ, Khatcheressian J, Lyman GH, Ozer H, Arm<strong>it</strong>age JO, Balducci L, Bennett CL, Cantor SB, CrawfordJ, Cross SJ, Demetri G, Desch CE, Pizzo PA, Schiffer CA, Schwartzberg L, Somerfield MR, Somlo G, WadeJC, Wade JL, Winn RJ, Wozniak AJ, Wolff AC: 2006 update of recommendations for the use of wh<strong>it</strong>e bloodcell growth factors: an evidence-based clinical practice guideline. J Clin Oncol, 24: 3187-3205, 2006.66. De Santis M, Becherer A, Bokemeyer C, Stoiber F, Oechsle K, Sellner F, Lang A, Kletter K, Dohmen BM,D<strong>it</strong>trich C, Pont J: 2-18fluoro-deoxy-D-glucose pos<strong>it</strong>ron emission tomography is a reliable predictor for viabletumor in postchemotherapy seminoma: an update of the prospective multicentric SEMPET trial. J ClinOncol, 22: 1034-1039, 2004.67. De Giorgi U, Pupi A, Fiorentini G, Rosti G, Marangolo M: FDG-PET in the management of germ cell tumor.Ann Oncol, 16(Suppl 4): iv90-iv94, 2005.68. Lewis DA, Tann M, Kesler K, McCool A, Foster RS, Einhorn LH: Pos<strong>it</strong>ron Emission Tomography Scans inPostchemotherapy Seminoma Patients W<strong>it</strong>h Residual Masses: A Retrospective Review From IndianaUnivers<strong>it</strong>y Hosp<strong>it</strong>al. J Clin Oncol, 24: e54 - e55, 2006.69. Hendry WF, A'Hern RP, Hetherington JW, Peckham MJ, Dearnaley DP, Horwich A: Para-aortic lymphadenectomyafter chemotherapy for metastatic non-seminomatous germ cell tumors: prognostic value and therapeuticbenef<strong>it</strong>, Br J Urol, 71: 208-213, 1993.70. Tekgul S, Özen HA, Celebi I, Ozgu I, Ergen A, Demircin M, Remzi D: Postchemotherapeutic surgery formetastatic testicular germ cell tumors: results of extended primary chemotherapy and lim<strong>it</strong>ed surgery.Urology, 43: 349-354, 1994.71. Fizazi K, Tjulandin S, Salvioni R, Germà-Lluch JR, Bouzy J, Ragan D, Bokemeyer C, Gerl A, Fléchon A, de BonoJS, Stenning S, Horwich A, Pont J, Albers P, De Giorgi U, Bower M, Bulanov A, Pizzocaro G, Aparicio J, NicholsCR, Théodore C, Hartmann JT, Schmoll HJ, Kaye SB, Culine S, Droz JP, Mahé C: Viable malignant cells afterprimary chemotherapy for disseminated nonseminomatous germ cell tumors: prognostic factors and role ofpostsurgery chemotherapy-results from an international study group, J Clin Oncol, 19: 2647-2657, 2001.72. Fizazi K, Oldenburg J, Dunant A, Chen I, Salvioni R, Hartmann JT, De Santis M, Daugaard G, Flechon A, DeGiorgi U, Tjulandin S, Schmoll HJ, Bouzy J, Fossa SD, Fromont G: Assessing prognosis and optimizing treatmentin patients w<strong>it</strong>h post-chemotherapy viable non-seminomatous germ-cell tumors (NSGCT): results ofthe sCR2 International study. Ann Oncol, (in press)73. Loehrer PJ, Gonin R, Nichols CR, Weathers T, Einhorn LH: Vinblastine plus iofosfamide plus cisplatin as in<strong>it</strong>ialsalvage therapy in recurrent germ cell tumor, J Clin Oncol 16: 2500-2504, 1998.74. Beyer J, Rick O, Siegert W, Bokemeyer C: Salvage chemotherapy in relapsed germ cell tumor. Word J Urol,19: 90-93, 2001.75. Kondagunta GV, Motzer RJ: Chemotherapy for advanced germ cell tumors. J Clin Oncol, 24: 5493-5502, 2006.76. Fossa SD, Stenning SP, Gerl A, Horwich A, Clark PI, Wilkinson PM, Jones WG, Williams MV, Oliver RT,Newlands ES, Mead GM, Cullen MH, Kaye SB, Rustin GJ, Cook PA: Prognostic factors in patients progressingafter cisplatin-based chemotherapy for malignant non-seminomatous germ cell tumours. Br J Cancer,80: 1392-1399, 1999.215
- Page 3:
Linee GuidaComitato SIU Linee Guida
- Page 6 and 7:
• Roberto SalvioniUrologia, Fonda
- Page 8 and 9:
NEOPLASIA DELLA VESCICA INFILTRANTE
- Page 11:
INDICELINEE GUIDA 20091. INFERTILIT
- Page 15:
INDICE1. EPIDEMIOLOGIA E FATTORI DI
- Page 18 and 19:
La definizione d'infertilità da fa
- Page 20 and 21:
gli ormoni prodotti dalle cellule d
- Page 22 and 23:
11. Gandini L, Lombardo F, Lenzi A,
- Page 25 and 26:
2. Approccioclinico-diagnosticoal m
- Page 27 and 28:
L’esame del liquido seminale comp
- Page 29 and 30:
La viscosità può essere sia norma
- Page 31 and 32:
Una motilità normale è definita d
- Page 33 and 34:
Nel caso in cui la percentuale di f
- Page 35 and 36:
Accanto ai fattori appena descritti
- Page 37 and 38:
integrare le indicazioni derivate d
- Page 39 and 40:
I mitocondri sono organelli richies
- Page 41 and 42:
Il Dna dello spermatozoo è organiz
- Page 43 and 44:
24. Sakkas D, Manicardi G, Bianchi
- Page 45 and 46:
Fig. 1Altri tipi di sonde sono quel
- Page 47 and 48:
Bibliografia1. Egozcue S, Blanco J,
- Page 49 and 50:
tropo). In quest'ultimo caso è nec
- Page 51 and 52:
tumore testicolare. Lo studio compl
- Page 53 and 54:
2.5 DIAGNOSTICA IMMUNOLOGICADai dat
- Page 55 and 56:
Bibliografia1. Coulam CB and Stern
- Page 57 and 58:
EFFETTO DELL’INFEZIONE SULLA QUAL
- Page 59 and 60:
tengono a 5 diversi generi: Alpha,
- Page 61 and 62:
Bibliografia1. Dinarello CA. Biolog
- Page 63 and 64:
2.7 DIAGNOSTICA ECOGRAFICANel perco
- Page 65 and 66:
cografia testicolare è particolarm
- Page 67 and 68:
Le dimensioni della prostata varian
- Page 69 and 70:
La misura meno soggetta a variazion
- Page 71 and 72:
2.8 DIAGNOSTICA CITOLOGICA ED AGOAS
- Page 73 and 74:
Fig. 3- spermatidi. Classicamente s
- Page 75 and 76:
Fig. 7- Quadri citologici nelle azo
- Page 77:
Tabella 2: Principali quadri citolo
- Page 80 and 81:
Come conseguenza diretta dell’alt
- Page 82 and 83:
Tutte le anomalie genetiche vengono
- Page 84 and 85:
Traslocazioni che coinvolgono i cro
- Page 86 and 87:
l’eiaculato o testicolari) per su
- Page 88 and 89:
cazione assoluta dal momento che la
- Page 91 and 92:
4. Quadri seminali4.1 AZOOSPERMIAL
- Page 93 and 94:
secretorie l’FSH è aumentato 2-8
- Page 95 and 96:
Fig. 3Pur essendo una rara causa di
- Page 97 and 98:
4.2 OLIGOZOOSPERMIA SEVERACon il te
- Page 99 and 100:
Studi geneticiL’esame del carioti
- Page 101 and 102:
Bibliografia1. Westlander G, Ekerho
- Page 103 and 104:
4.4 ASTENOZOOSPERMIANell’ambito d
- Page 105 and 106:
FLOW-CHART DIAGNOSTICA103
- Page 107 and 108:
5. Principali quadri cliniciassocia
- Page 109 and 110:
eflusso viene considerato patologic
- Page 111 and 112:
Attualmente una precisa indicazione
- Page 113 and 114:
5.2 CRIPTORCHIDISMODefinizione e cl
- Page 115 and 116:
Cenni di embriologiaLa discesa del
- Page 117 and 118:
- FG syndrome- del 22q11.2- del 1p3
- Page 119 and 120:
gressivi danni all’epitelio semin
- Page 121 and 122:
Ostruzioni epididimaliLe ostruzioni
- Page 123 and 124:
o acquisite (cisti) a carico dei va
- Page 125 and 126:
5.4 INFEZIONI DELLE GHIANDOLE SESSU
- Page 127 and 128:
disturbi disurici (6%) 4 . Nelle fo
- Page 129 and 130:
EpididimitiL’infiammazione dell
- Page 131 and 132:
6. L’approccio terapeuticoal masc
- Page 133 and 134:
Bibliografia1. Matsumoto AM. Hormon
- Page 135 and 136:
Terapia androgenicaL’induzione ed
- Page 137 and 138:
Terapia con antiestrogeniIl raziona
- Page 139 and 140:
specificamente il perossido di idro
- Page 141 and 142:
6.3 TERAPIA CHIRURGICALa terapia ch
- Page 143 and 144:
7. Metodichedi crioconservazione7.1
- Page 145 and 146:
Nel congelamento lento, invece, il
- Page 147 and 148:
Le tecniche più utilizzate per il
- Page 149 and 150:
7.6 CONCLUSIONILa crioconservazione
- Page 151 and 152:
32. Desai N, Glavan D, Goldfarb J.
- Page 153 and 154:
8.Parametri seminalie tecniche di p
- Page 155 and 156:
Nei casi di bassa concentrazione sp
- Page 157 and 158:
Infertilità di eziologia sconosciu
- Page 159 and 160:
8.2 FECONDAZIONE IN VITRO CON EMBRI
- Page 161 and 162:
Condizioni non chiaramente legate a
- Page 163 and 164:
8.4 INIEZIONE INTRACITOPLASMATICA D
- Page 165 and 166: 23. Bartoov B, Berkovitz A, Eltes F
- Page 167 and 168: Capitolo 2I DISTURBI DELL'EIACULAZI
- Page 169 and 170: IntroduzioneLe disfunzioni eiaculat
- Page 171 and 172: 1. Eiaculazione PrecoceLa mancanza
- Page 173 and 174: mente il segnale da un neurone all'
- Page 175 and 176: demand sia con assunzione giornalie
- Page 177 and 178: Figura 1. Modificata da: CG. McMaho
- Page 179 and 180: 2. AneiaculazioneAlcune patologie p
- Page 181 and 182: 3. Anorgasmia edeiaculazione ritard
- Page 183 and 184: DIAGNOSILa diagnosi è innanzi tutt
- Page 185 and 186: Figura 2. Modificata da: CG. McMaho
- Page 187 and 188: 4. Eiaculazione RetrogradaDEFINIZIO
- Page 189 and 190: Bibliografia1. CG. McMahon. Disorde
- Page 191 and 192: 51. GM Colpi, G. Piediferro. Ritard
- Page 193 and 194: Capitolo 3NEOPLASIA DEL TESTICOLOR.
- Page 195 and 196: IntroduzioneI tumori a cellule germ
- Page 197 and 198: Linee guidapratiche IGG per pazient
- Page 199 and 200: orchiectomia (nel seminoma puro dev
- Page 201 and 202: stazioni linfonodali para-aortiche
- Page 203 and 204: NON-SEMINOMA STADIO ILa prognosi de
- Page 205 and 206: Se la RPLND è eseguita senza la st
- Page 207 and 208: Non-seminoma stadio clinico IIA mar
- Page 209 and 210: Anche il trattamento di prima linea
- Page 211 and 212: te investigata nei GCT 77,78 . Dopo
- Page 213 and 214: filattica nei casi a basso potenzia
- Page 215: 20. Giwercman A, Cantell L, Marks A
- Page 219 and 220: Capitolo 4NEOPLASIA DELLA VESCICAIN
- Page 221 and 222: IntroduzioneAl momento della diagno
- Page 223 and 224: Diagnosi e stadiazioneL'imaging gio
- Page 225 and 226: • In previsione di un trattamento
- Page 227 and 228: Opzioni terapeuticheLa cistectomia
- Page 229 and 230: ne nei tessuti e nell'urina anche d
- Page 231 and 232: ciata a terapia endovescicale, gene
- Page 233 and 234: l'uretra prostatica) (Cresswell, 20
- Page 235 and 236: ti variabili, come l'età del pazie
- Page 237 and 238: BibliografiaAbol-Enein H, Ghoneim M
- Page 239 and 240: Hardt J, Filipas D, Hohenfellner R,
- Page 241 and 242: Rosenberg JE, Carroll PR, Small EJ.
- Page 243 and 244: Tumore vescicale metastaticoe Chemi
- Page 245 and 246: CATEGORIE PECULIARI DI PAZIENTIPazi
- Page 247 and 248: BibliografiaBamias A, Efstathiou E,
- Page 249 and 250: 247
- Page 251 and 252: Capitolo 5CALCOLOSI DELL’URETEREC
- Page 253 and 254: Fattori da considerarenella scelta
- Page 255 and 256: Opzioni terapeuticheper il paziente
- Page 257 and 258: • Rivalutazione clinica ematochim
- Page 259 and 260: C3. URETEROLITOTOMIA A CIELO APERTO
- Page 261 and 262: BibliografiaQueste linee guida sono
- Page 263 and 264: Capitolo 6CALCOLOSI RENALEF. Zatton
- Page 265 and 266: 1. Criteri di utilizzoLe presenti l
- Page 267 and 268:
2. IntroduzioneL’urolitiasi occup
- Page 269 and 270:
3. Classificazionee fattori di risc
- Page 271 and 272:
4. Diagnostica strumentalee di labo
- Page 273 and 274:
Bibliografia1. Smith RC, Rosenfield
- Page 275 and 276:
5. Ricerca di fattori rischioper ur
- Page 277 and 278:
sovrasaturazione con ossalato di ca
- Page 279 and 280:
Bibliografia1. Tiselius HG, Alken P
- Page 281 and 282:
6. Massa litiasicaLa “massa litia
- Page 283 and 284:
7.Trattamentodella colica renaleLe
- Page 285 and 286:
8. Indicazioni perla rimozione diun
- Page 287 and 288:
9. Procedure perla rimozione deical
- Page 289 and 290:
I fattori che influenzano l’esito
- Page 291 and 292:
of stones from the urinary tract. J
- Page 293 and 294:
9.2 LITOTRISSIA PERCUTANEA (PNL)La
- Page 295 and 296:
Bibliografia1. Kim SC, Kuo RL, Ling
- Page 297 and 298:
Per il trattamento di calcoli renal
- Page 299 and 300:
20. Grasso M, Chalik Y. Principles
- Page 301 and 302:
9.4 CHIRURGIA “A CIELO APERTO”I
- Page 303 and 304:
9.5 LAPAROSCOPIACon l’acquisizion
- Page 305 and 306:
9.6 CHEMIOLISI PERCUTANEALa chemiol
- Page 307 and 308:
9.7 GESTIONE DI PROBLEMI “PARTICO
- Page 309 and 310:
10. Inquadramentometabolico dellane
- Page 311 and 312:
A. Acidosi tubulare distale complet
- Page 313 and 314:
CALCOLOSI CALCICA (continua Tabella
- Page 315 and 316:
TRATTAMENTO PREVENTIVO NELLA CALCOL
- Page 317 and 318:
CALCOLOSI CALCICA (continua tabella
- Page 319 and 320:
11. Sinossi delle principaliraccoma
- Page 321 and 322:
Nel caso in cui pazienti con calcol
- Page 323 and 324:
11.5 DIAGNOSI DELL’UROLITIASI IN
- Page 325 and 326:
Tabella 28. Raccomandazioni per il
- Page 327 and 328:
11.11 GESTIONE DEI PAZIENTI CON CAL
- Page 329 and 330:
AbbreviazioniCKD:CY:ESWL:GR:INF:KDO
- Page 331 and 332:
Capitolo 7STENOSI DELL’URETRA:DIA
- Page 333 and 334:
AnatomiaL’uretra maschile non è
- Page 335 and 336:
Il suo scopo è fornire informazion
- Page 337 and 338:
dono al collassamento sono cadute i
- Page 339 and 340:
TESSUTI DI SOSTITUZIONE URETRALELa
- Page 341 and 342:
Bibliografia1 Jordan GH.Gestione de
- Page 343 and 344:
341
- Page 345 and 346:
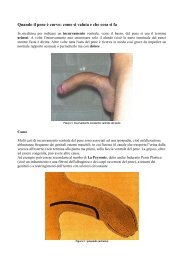
Capitolo 8CARCINOMA DEL PENEG. Pizz
- Page 347 and 348:
INDICE1. FONTI UTILIZZATE pag 3472.
- Page 349 and 350:
1. Fonti utilizzateLa struttura por
- Page 351 and 352:
2. Epidemiologia ed EziologiaIl car
- Page 353 and 354:
3. Classificazione e Patologia3.1 C
- Page 355 and 356:
4. Diagnosi e StadiazioneUna stadia
- Page 357 and 358:
Tuttavia, tutti questi metodi non r
- Page 359 and 360:
5. Trattamento5.1 TUMORE PRIMITIVO5
- Page 361 and 362:
60 Gy 46 . La brachiterapia (BRT) h
- Page 363 and 364:
5.2.4 Trattamento dei pazienti con
- Page 365 and 366:
6. Follow-upLo scopo del follow-up
- Page 367 and 368:
7. Qualità della vita7.1 SESSUALIT
- Page 369 and 370:
ConclusioniAllo stato attuale, circ
- Page 371 and 372:
TabelleTabella 1. Livelli di eviden
- Page 373 and 374:
Tabella 4. Classificazione TNM del
- Page 375 and 376:
Tabella 8. Classificazione anatomo-
- Page 377 and 378:
Bibliografia1 Pizzocaro G, Algaba F
- Page 379 and 380:
51 Culkin DJ, Beer TM. Advanced pen
- Page 381 and 382:
APPENDICECARCINOMA DELLA PROSTATAag
- Page 383 and 384:
Screening nel tumoredella prostataL
- Page 385 and 386:
Bibliografia1. Oliver SE, May MT, G
- Page 387 and 388:
Prevenzione deltumore prostaticoLe
- Page 389 and 390:
INDICE LINEE GUIDA • EDIZIONE SET