TROLL 9500 Operator's Manual - Geotech Environmental Equipment
TROLL 9500 Operator's Manual - Geotech Environmental Equipment
TROLL 9500 Operator's Manual - Geotech Environmental Equipment
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
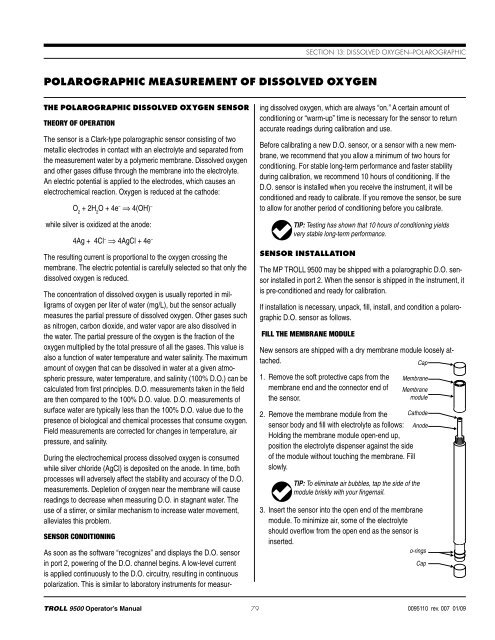
Section 13: Dissolved Oxygen—PolarographicPolarographic Measurement of Dissolved OxygenThe Polarographic Dissolved Oxygen SensorTheory of OperationThe sensor is a Clark-type polarographic sensor consisting of twometallic electrodes in contact with an electrolyte and separated fromthe measurement water by a polymeric membrane. Dissolved oxygenand other gases diffuse through the membrane into the electrolyte.An electric potential is applied to the electrodes, which causes anelectrochemical reaction. Oxygen is reduced at the cathode:O 2+ 2H 2O + 4e – ⇒ 4(OH) –while silver is oxidized at the anode:4Ag + 4Cl – ⇒ 4AgCl + 4e –The resulting current is proportional to the oxygen crossing themembrane. The electric potential is carefully selected so that only thedissolved oxygen is reduced.The concentration of dissolved oxygen is usually reported in milligramsof oxygen per liter of water (mg/L), but the sensor actuallymeasures the partial pressure of dissolved oxygen. Other gases suchas nitrogen, carbon dioxide, and water vapor are also dissolved inthe water. The partial pressure of the oxygen is the fraction of theoxygen multiplied by the total pressure of all the gases. This value isalso a function of water temperature and water salinity. The maximumamount of oxygen that can be dissolved in water at a given atmosphericpressure, water temperature, and salinity (100% D.O.) can becalculated from first principles. D.O. measurements taken in the fieldare then compared to the 100% D.O. value. D.O. measurements ofsurface water are typically less than the 100% D.O. value due to thepresence of biological and chemical processes that consume oxygen.Field measurements are corrected for changes in temperature, airpressure, and salinity.During the electrochemical process dissolved oxygen is consumedwhile silver chloride (AgCl) is deposited on the anode. In time, bothprocesses will adversely affect the stability and accuracy of the D.O.measurements. Depletion of oxygen near the membrane will causereadings to decrease when measuring D.O. in stagnant water. Theuse of a stirrer, or similar mechanism to increase water movement,alleviates this problem.Sensor ConditioningAs soon as the software “recognizes” and displays the D.O. sensorin port 2, powering of the D.O. channel begins. A low-level currentis applied continuously to the D.O. circuitry, resulting in continuouspolarization. This is similar to laboratory instruments for measuringdissolved oxygen, which are always “on.” A certain amount ofconditioning or “warm-up” time is necessary for the sensor to returnaccurate readings during calibration and use.Before calibrating a new D.O. sensor, or a sensor with a new membrane,we recommend that you allow a minimum of two hours forconditioning. For stable long-term performance and faster stabilityduring calibration, we recommend 10 hours of conditioning. If theD.O. sensor is installed when you receive the instrument, it will beconditioned and ready to calibrate. If you remove the sensor, be sureto allow for another period of conditioning before you calibrate.TIP: Testing has shown that 10 hours of conditioning yieldsvery stable long-term performance.Sensor InstallationThe MP <strong>TROLL</strong> <strong>9500</strong> may be shipped with a polarographic D.O. sensorinstalled in port 2. When the sensor is shipped in the instrument, itis pre-conditioned and ready for calibration.If installation is necessary, unpack, fill, install, and condition a polarographicD.O. sensor as follows.Fill the Membrane ModuleNew sensors are shipped with a dry membrane module loosely attached.Cap1. Remove the soft protective caps from themembrane end and the connector end ofthe sensor.MembraneMembranemodule2. Remove the membrane module from the Cathodesensor body and fill with electrolyte as follows: AnodeHolding the membrane module open-end up,position the electrolyte dispenser against the sideof the module without touching the membrane. Fillslowly.TIP: To eliminate air bubbles, tap the side of themodule briskly with your fingernail.3. Insert the sensor into the open end of the membranemodule. To minimize air, some of the electrolyteshould overflow from the open end as the sensor isinserted.o-ringsCap<strong>TROLL</strong> <strong>9500</strong> Operator’s <strong>Manual</strong> 790095110 rev. 007 01/09