Thermo Scientific TVA-1000B Instruction Manual - Geotech ...
Thermo Scientific TVA-1000B Instruction Manual - Geotech ...
Thermo Scientific TVA-1000B Instruction Manual - Geotech ...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
Theory of Operation<br />
Flame Ionization Detection (FID)<br />
A Flame Ionization Detector (FID) measures organic compounds by utilizing a flame produced<br />
by the combustion of hydrogen and air. When hydrocarbons in the sample are introduced<br />
to the detection zone, ions are produced by the following reaction:<br />
RH + O → RHO + + e – → H 2 O + CO 2<br />
where<br />
R = carbon compound<br />
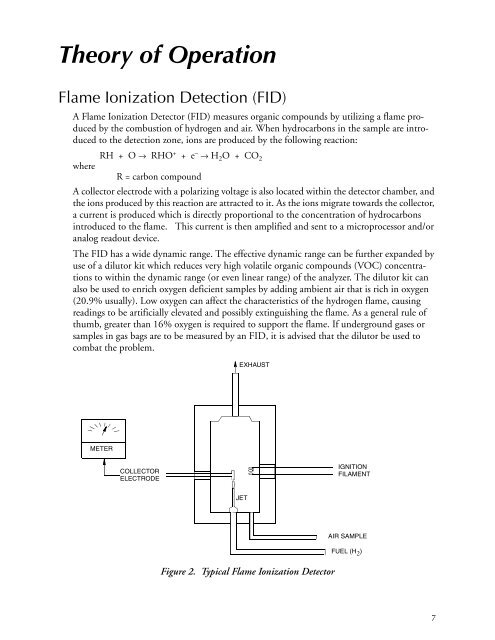
A collector electrode with a polarizing voltage is also located within the detector chamber, and<br />
the ions produced by this reaction are attracted to it. As the ions migrate towards the collector,<br />
a current is produced which is directly proportional to the concentration of hydrocarbons<br />
introduced to the flame. This current is then amplified and sent to a microprocessor and/or<br />
analog readout device.<br />
The FID has a wide dynamic range. The effective dynamic range can be further expanded by<br />
use of a dilutor kit which reduces very high volatile organic compounds (VOC) concentrations<br />
to within the dynamic range (or even linear range) of the analyzer. The dilutor kit can<br />
also be used to enrich oxygen deficient samples by adding ambient air that is rich in oxygen<br />
(20.9% usually). Low oxygen can affect the characteristics of the hydrogen flame, causing<br />
readings to be artificially elevated and possibly extinguishing the flame. As a general rule of<br />
thumb, greater than 16% oxygen is required to support the flame. If underground gases or<br />
samples in gas bags are to be measured by an FID, it is advised that the dilutor be used to<br />
combat the problem.<br />
EXHAUST<br />
METER<br />
COLLECTOR<br />
ELECTRODE<br />
IGNITION<br />
FILAMENT<br />
JET<br />
Figure 2. Typical Flame Ionization Detector<br />
AIR SAMPLE<br />
FUEL (H 2 )<br />
7