Climate Change and the European Water Dimension - Agri ...
Climate Change and the European Water Dimension - Agri ...
Climate Change and the European Water Dimension - Agri ...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
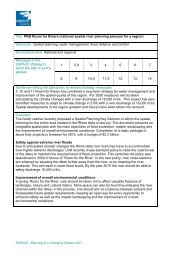
Table VI.D.2.– Mercury depletion (%) as a function of relative humidity<br />
(rh) <strong>and</strong> O3 concentration (O3 concentrations were derived from IPCC<br />
(2001) WG-1 scenarios).<br />
st<strong>and</strong>ards are often exceeded) <strong>and</strong> OH proves to be a major Hg 0 (g) oxidant even in<br />
<strong>the</strong> MBL. The impact of increasing O3 concentrations in <strong>the</strong> atmosphere, including<br />
<strong>the</strong> boundary layer seen in some IPCC modelling scenarios, would <strong>the</strong>refore have an<br />
influence on <strong>the</strong> atmospheric oxidation rate of Hg 0 (g), which in turn would influence<br />
Hg deposition both in terms of dry <strong>and</strong> wet deposition. The nature of this influence is<br />
not however entirely clear. Over <strong>the</strong> l<strong>and</strong> Hg oxidation would certainly occur more<br />
rapidly, although both relative humidity (which influences OH concentration) <strong>and</strong> O3<br />
concentration play a part as seen in Table VI.D.II. The figures reported in Table<br />
VI.D.2 were obtained using a version of <strong>the</strong> Chemical Balance Model-IV (CMB-IV) to<br />
which atmospheric Hg chemistry has been added.<br />
Due to its relative involatility, Hg(II)(g) produced in <strong>the</strong> atmosphere is readily<br />
scavenged by rain <strong>and</strong> also by particulate matter. If O3 <strong>and</strong> particulate matter<br />
concentrations increase, <strong>the</strong> probability is that continental air would contain more<br />
oxidised Hg(II)(g) <strong>and</strong> Hg associated with particulates than previously, especially if <strong>the</strong><br />
air were humid. Deposition of Hg would <strong>the</strong>refore occur closer to sources, or would<br />
occur where dryer continental air meets more humid air. Thus <strong>the</strong>re is <strong>the</strong> possibility<br />
that Hg deposition would increase substantially in coastal areas. Hg sources located<br />
near coasts could provide much more local contamination than has so far been <strong>the</strong><br />
case.<br />
The cycling of Hg in <strong>the</strong> MBL is not yet entirely understood, certainly Hg 0 (g) is<br />
oxidised quite rapidly under conditions favouring <strong>the</strong> release of reactive halogen<br />
compounds from sea salt aerosols, <strong>and</strong> it has been suggested that changes in global<br />
wind fields as a result of climate change would alter <strong>the</strong> distribution of <strong>the</strong> acid gases<br />
which promote halogen activation <strong>and</strong> also tend to result in higher production rates of<br />
sea salt aerosol. This would increase <strong>the</strong> rate of Hg 0 (g) oxidation in <strong>the</strong> MBL.<br />
Interestingly though a concurrent increase of <strong>the</strong> boundary layer O3 concentration<br />
would actually contrast this increase because higher O3 would produce higher HO2<br />
which is <strong>the</strong> primary sink for active Br compounds in <strong>the</strong> MBL. To test this hypo<strong>the</strong>sis<br />
<strong>the</strong> AMCOTS box model (Hedgecock <strong>and</strong> Pirrone 2001; 2003) which has already<br />
been used to study <strong>the</strong> lifetime of Hg 0 (g) in <strong>the</strong> MBL has been run with higher than<br />
usual O3 concentrations (as suggested in IPCC (2001) WG-1 scenarios). Using<br />
typical seasonal cloud cover <strong>and</strong> air temperature values for <strong>the</strong> three latitudes used,<br />
increasing <strong>the</strong> initial O3 concentration in <strong>the</strong> model, actually results in less Hg 0 (g)<br />
depletion in most instances.<br />
Why <strong>the</strong> MBL is important in <strong>the</strong> Hg Cycle?<br />
The fact that in spite of rapid oxidation in <strong>the</strong> MBL <strong>the</strong> background Hg 0 (g)<br />
concentration, at least hemispherically, points to an extremely dynamic exchange of<br />
Hg between <strong>the</strong> MBL <strong>and</strong> marine waters. Unfortunately not all <strong>the</strong> processes that<br />
regulate this exchange are at all well understood, but <strong>the</strong> perturbation of <strong>the</strong><br />
processes that contribute could result in an imbalance, leading to far greater Hg input<br />
into coastal <strong>and</strong> marine waters.<br />
194<br />
O3 concentration<br />
% Hg 0 (g) depletion after 7 days 30 ppb 60 ppb 80 ppb<br />
10% r.h. 12.5 18 21<br />
40% r.h. 19.5 28 33<br />
70% r.h. 24 34 40