Sudbø-saken UiO 2006.pdf - De nasjonale forskningsetiske komiteer
Sudbø-saken UiO 2006.pdf - De nasjonale forskningsetiske komiteer
Sudbø-saken UiO 2006.pdf - De nasjonale forskningsetiske komiteer
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
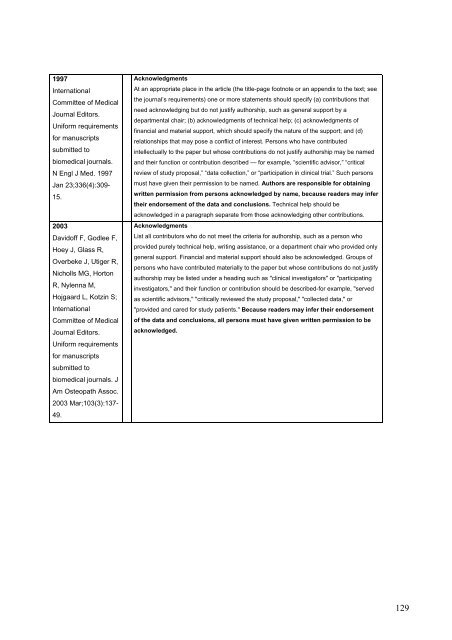
1997<br />
International<br />
Committee of Medical<br />
Journal Editors.<br />
Uniform requirements<br />
for manuscripts<br />
submitted to<br />
biomedical journals.<br />
N Engl J Med. 1997<br />
Jan 23;336(4):309-<br />
15.<br />
2003<br />
Davidoff F, Godlee F,<br />
Hoey J, Glass R,<br />
Overbeke J, Utiger R,<br />
Nicholls MG, Horton<br />
R, Nylenna M,<br />
Hojgaard L, Kotzin S;<br />
International<br />
Committee of Medical<br />
Journal Editors.<br />
Uniform requirements<br />
for manuscripts<br />
submitted to<br />
biomedical journals. J<br />
Am Osteopath Assoc.<br />
2003 Mar;103(3):137-<br />
49.<br />
Acknowledgments<br />
At an appropriate place in the article (the title-page footnote or an appendix to the text; see<br />
the journal’s requirements) one or more statements should specify (a) contributions that<br />
need acknowledging but do not justify authorship, such as general support by a<br />
departmental chair; (b) acknowledgments of technical help; (c) acknowledgments of<br />
financial and material support, which should specify the nature of the support; and (d)<br />
relationships that may pose a conflict of interest. Persons who have contributed<br />
intellectually to the paper but whose contributions do not justify authorship may be named<br />
and their function or contribution described — for example, “scientific advisor,” “critical<br />
review of study proposal,” “data collection,” or “participation in clinical trial.” Such persons<br />
must have given their permission to be named. Authors are responsible for obtaining<br />
written permission from persons acknowledged by name, because readers may infer<br />
their endorsement of the data and conclusions. Technical help should be<br />
acknowledged in a paragraph separate from those acknowledging other contributions.<br />
Acknowledgments<br />
List all contributors who do not meet the criteria for authorship, such as a person who<br />
provided purely technical help, writing assistance, or a department chair who provided only<br />
general support. Financial and material support should also be acknowledged. Groups of<br />
persons who have contributed materially to the paper but whose contributions do not justify<br />
authorship may be listed under a heading such as "clinical investigators" or "participating<br />
investigators," and their function or contribution should be described-for example, "served<br />
as scientific advisors," "critically reviewed the study proposal," "collected data," or<br />
"provided and cared for study patients." Because readers may infer their endorsement<br />
of the data and conclusions, all persons must have given written permission to be<br />
acknowledged.<br />
129