Air quality expert group - Fine particulate matter (PM2.5) in ... - Defra
Air quality expert group - Fine particulate matter (PM2.5) in ... - Defra
Air quality expert group - Fine particulate matter (PM2.5) in ... - Defra
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
<strong>PM2.5</strong> <strong>in</strong> the UK<br />
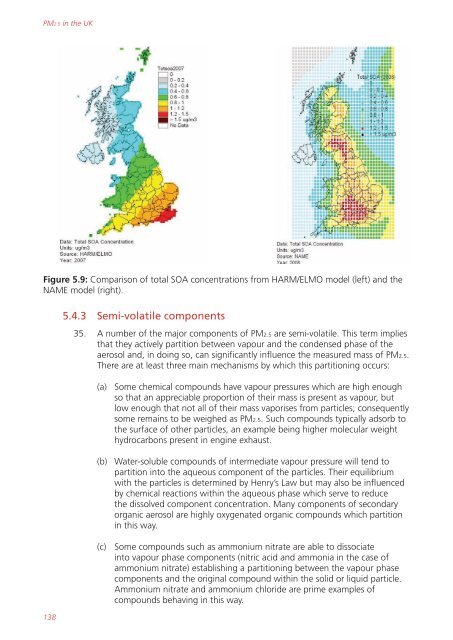
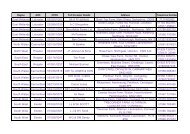
Figure 5.9: Comparison of total SOA concentrations from HARM/ELMO model (left) and the<br />
NAME model (right).<br />
138<br />
5.4.3 Semi-volatile components<br />
35. A number of the major components of <strong>PM2.5</strong> are semi-volatile. This term implies<br />
that they actively partition between vapour and the condensed phase of the<br />
aerosol and, <strong>in</strong> do<strong>in</strong>g so, can significantly <strong>in</strong>fluence the measured mass of <strong>PM2.5</strong>.<br />
There are at least three ma<strong>in</strong> mechanisms by which this partition<strong>in</strong>g occurs:<br />
(a) Some chemical compounds have vapour pressures which are high enough<br />
so that an appreciable proportion of their mass is present as vapour, but<br />
low enough that not all of their mass vaporises from particles; consequently<br />
some rema<strong>in</strong>s to be weighed as <strong>PM2.5</strong>. Such compounds typically adsorb to<br />
the surface of other particles, an example be<strong>in</strong>g higher molecular weight<br />
hydrocarbons present <strong>in</strong> eng<strong>in</strong>e exhaust.<br />
(b) Water-soluble compounds of <strong>in</strong>termediate vapour pressure will tend to<br />
partition <strong>in</strong>to the aqueous component of the particles. Their equilibrium<br />
with the particles is determ<strong>in</strong>ed by Henry’s Law but may also be <strong>in</strong>fluenced<br />
by chemical reactions with<strong>in</strong> the aqueous phase which serve to reduce<br />
the dissolved component concentration. Many components of secondary<br />
organic aerosol are highly oxygenated organic compounds which partition<br />
<strong>in</strong> this way.<br />
(c) Some compounds such as ammonium nitrate are able to dissociate<br />
<strong>in</strong>to vapour phase components (nitric acid and ammonia <strong>in</strong> the case of<br />
ammonium nitrate) establish<strong>in</strong>g a partition<strong>in</strong>g between the vapour phase<br />
components and the orig<strong>in</strong>al compound with<strong>in</strong> the solid or liquid particle.<br />
Ammonium nitrate and ammonium chloride are prime examples of<br />
compounds behav<strong>in</strong>g <strong>in</strong> this way.