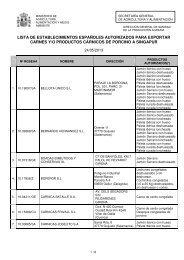
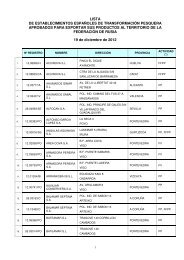
Зарегистрировано в Минюсте РФ 22 марта 2002 г - Cexgan
Зарегистрировано в Минюсте РФ 22 марта 2002 г - Cexgan
Зарегистрировано в Минюсте РФ 22 марта 2002 г - Cexgan
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
а) examination of technological instructions for production (hereinafter referred to as the TI), which<br />
establish requirements for the processes of manufacturing, inspection, packaging, marking of products at a<br />
particular plant, including drafts of label prints on consumer packagings (package inserts, instructions for use)<br />
as well as draft production plans with production control programme;<br />
b) selective laboratory examinations of samples of raw materials and foodstuffs from the pilot batches<br />
of products;<br />
c) examination of production conditions (at plants manufacturing viable GMM or GMA or using viable<br />
GMM or GMA in the technological process of food production).<br />
5.8.4. Presence of requirements and parameters governing the use of GMM or GMA in the technological<br />
process shall be controlled in the process of examination of TI for a particular type of food product:<br />
a) in the section 'Technical Requirements' - information about the presence or absence in the raw<br />
material and components of this type of product, their generic and specific belonging;<br />
b) in the section 'Methods of Control' - a description of methods of analysis (references to approved<br />
methods) of microorganisms of controlled technological microflora which should contain 1 g of food products<br />
and methods of identification of generic and specific belonging (in cases provided for by the regulatory and<br />
technical documentation - the lack of living cells of strains-producers); in products derived from / or with the<br />
use of GMM - the lack of genes of transmissible antibiotic resistance (selective markers of antibiotic<br />
resistance); if necessary - the target genes of GMM, products of expression of target genes of GMM, as well<br />
as other methods of analysis, allowing to confirm the type and properties of GMM or GMA contained in the<br />
product;<br />
c) in the section "Marking" and on the label of consumer packaging - information about the relevance<br />
of the product and GMM and information for consumers about the presence of GMM in the given type of<br />
product, taking into account Clause 2.18 of this Sanitary Rules;<br />
d) regarding production preparation - description of the production control system, including incoming<br />
inspection of raw materials and components (presence of sanitary-epidemiological conclusions and other<br />
documents confirming their relation to the GMM and GMA), laboratory control (regarding the absence or<br />
presence of GMM (GMA) and / or selective markers of GMM, if necessary - target genes of GMM, products of<br />
expression of target genes of GMM); at plants producing strains-producers of food substances - additional<br />
control over production conditions, control over working area air, surfaces and equipment - for the presence of<br />
living cells of GMM (GMA) producers.<br />
ConsultantPlus: note:<br />
The official text of the document obviously contains a misprint - Clause 5.7 has no Sub-clauses.<br />
Apparently Sub-clause 'b' of Clause 5.6 is meant.<br />
5.8.5. While monitoring the production process food samples shall be selected from the pilot batch and<br />
laboratory test shall be carried out for determination of the presence of GMM and / or selective markers of<br />
GMM, and if necessary - additional tests of products and raw materials in accordance with Clause 5.7 'b'.<br />
5.8.6. Production examination shall be carried out by means of:<br />
а) conformity assessment of business units (laboratories, starter shops, shops or sites), working with<br />
living fermentation, starter, probiotic, yeast cultures and strains-producers of food substances and food<br />
additives, with the requirements of sanitary rules for the corresponding industries and, if necessary (at plants,<br />
generating strains-producers) - with the requirements of sanitary rules for safety of working with<br />
microorganisms and for procedures of registration, storage, transfer and transportation of microorganisms;<br />
b) examination of programme of production control of products performed at the manufacturing plant<br />
monitoring control of GMO and GMA regarding meeting the requirements of sanitary rules for organization and<br />
exercising production control over compliance with sanitary rules and implementation of sanitary and epidemic<br />
(prevention) activities;<br />
c) examination of documentation for raw materials and components, food products, which are<br />
in production and expedition, regarding the records about the presence of GMM in technical specifications for<br />
the ingredient composition, in label prints and in quality and safety certificate for finished product.<br />
5.9. When carrying out state sanitary and epidemiological control over food products derived from / or with<br />
the use of GMM and GMA, during the processes of production, storage, transportation and sale it is required to<br />
check the presence of regulatory and technical documents for specific types of products (standards, technical<br />
specifications, composition, specifications for imported products), certificates of state registration and sanitaryepidemiological<br />
conclusions on conformity with sanitary rules, issued in the established manner.<br />
5.9.1. The sanitary and epidemiological examination of food products derived from / or with the use of GMM<br />
and GMA, during the processes of production, storage, transportation and sale includes selective laboratory<br />
tests for identification of the presence in the product of GMM and / or selective markers of GMM, and if<br />
necessary - additional product and material testing in accordance with Clause 5.6 'b'.<br />
5.9.2. State sanitary and epidemiological control includes examination of documents for raw materials and<br />
components, food products, which are located at the site under control and intended for storage, transportation<br />
and sale, regarding the information about the presence of GMM in the technical documents, on the label, as<br />
well as in the quality and safety certificate for the batch of finished products.