xxiii Ïανελληνιο ÏÏ Î½ÎµÎ´Ïιο ÏÏ ÏÎ¹ÎºÎ·Ï ÏÏεÏÎµÎ±Ï ÎºÎ±ÏαÏÏαÏÎ·Ï & εÏιÏÏÎ·Î¼Î·Ï ...
xxiii Ïανελληνιο ÏÏ Î½ÎµÎ´Ïιο ÏÏ ÏÎ¹ÎºÎ·Ï ÏÏεÏÎµÎ±Ï ÎºÎ±ÏαÏÏαÏÎ·Ï & εÏιÏÏÎ·Î¼Î·Ï ...
xxiii Ïανελληνιο ÏÏ Î½ÎµÎ´Ïιο ÏÏ ÏÎ¹ÎºÎ·Ï ÏÏεÏÎµÎ±Ï ÎºÎ±ÏαÏÏαÏÎ·Ï & εÏιÏÏÎ·Î¼Î·Ï ...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
Ba Adsorption on an Oxygen Chemisorbed O(2×1)/Ni(110) Surface<br />
D. Vlachos, S.D. Foulias and M. Kamaratos<br />
Department of Physics, University of Ioannina, PO Box 1186, GR-451 10, Ioannina, Greece<br />
d.vlachos@cc.uoi.gr<br />
Abstract<br />
In this work we study Ba adsorption at submonolayer and monolayer coverages on an oxygen chemisorbed<br />
O(2×1)/Ni(110) surface, by means of Auger electron spectroscopy, low energy electron diffraction and work function<br />
measurements. Ba adsorption results initially in an incomplete two dimensional barium oxide layer, BaO, due to the<br />
interaction of the Ba adatoms and the chemisorbed O atoms. A fraction of the first layer Ba atoms seems to be chemisorbed<br />
reacting directly with Ni atoms. At higher coverages, Ba approaches the metallic phase. The observed energy shifts of the Ba<br />
and BaO related low energy Auger transmission lines are attributed to both initial and final state effects, which are strongly<br />
dependent on the substrate where the Ba-O interaction takes place.<br />
1. Introduction<br />
Barium oxide, BaO, is quite an important material in modern technology mainly because of its low work function, electron<br />
emissivity and catalytic properties. Of special interest is the formation of ultra-thin films BaO on surfaces, where significant<br />
changes of the electronic and the physicochemical properties of the substrate are observed. Ultrathin films of BaO on metallic<br />
substrates of W and Pt, have given significant technological applications, in the fabrication of high current density cathodes<br />
[1,2], and in the design of modern catalysts for NO x compounds storage in exhaust emission from motor vehicles [3,4].<br />
To better understand the electronic and physicochemical properties of the BaO/substrate, it is important to study how the<br />
BaO overlayer starts developing on the surface from sub-monolayer to monolayer. Also, it is quite crucial to percieve the<br />
nature of the bonding between Ba and O atoms in BaO compound on surface. This is a controversial issue in the literature<br />
regarding the degree of the ionicity. In this work, we study the Ba-O interaction on a metallic surface by the adsorption of Ba<br />
on the oxygen chemisorbed O(2x1)/Ni(110) surface. Our intention is to investigate the Ba-O interaction on the surface from<br />
the very first stage of Ba adsorption and to study how the substrate affects this interaction.<br />
2. Experimental part<br />
The experiments were performed in an ultra high vacuum system (UHV) at base pressure of 10 -10 Torr. The system was<br />
equipped with Auger electron spectroscopy (AES), low energy electron diffraction (LEED), a quadrupole mass spectrometer<br />
(QMS) and a Kelvin probe for work function measurements (WF). The sample was a Ni(110) single crystal with dimensions<br />
1cm× 0.5cm× 0.1cm. Barium deposition was carried out by means of a commercial evaporation SAES Getters source at<br />
constant current 6.5 A in steps of 2 min. Assuming the sticking coefficient of Ba on the oxygen predeposited nickel to be<br />
constant and equal to that on the clean surface, the coverage could be estimated in monolayers (~0.1ML/2min) by combining<br />
our results with previous AES, LEED, TDS and WF results [5]. The oxygen adsorption took place by supplying molecular<br />
oxygen of purity 99.998 vol% into the experimental chamber through a leak valve. The oxygen exposure is counted in<br />
Langmuirs, (L) where 1L=1× 10 -6 Torr.s.<br />
AP-PH (arb. units)<br />
1.4<br />
1.2<br />
1.0<br />
0.8<br />
0.6<br />
0.4<br />
0.2<br />
Ba/O(2x1)/Ni(110) Ni(848eV)<br />
Ba(590eV)x2<br />
ΔΦ (eV)<br />
0.0<br />
-4.0<br />
0 5 10 15 20 25 30 35 40 45<br />
Ba deposition (min)<br />
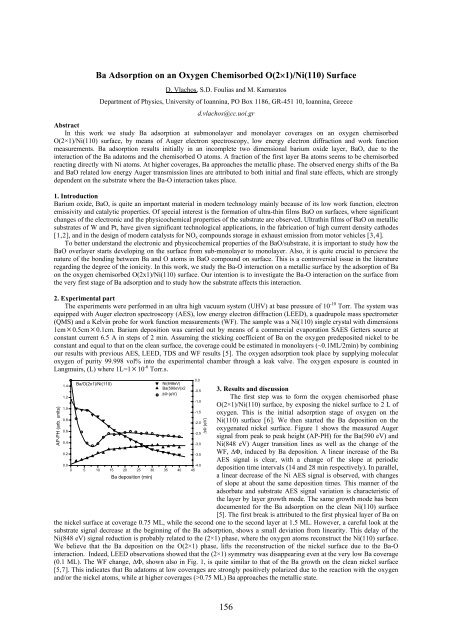
Figure 1 The AP-PHs of the Ba(590 eV) and Ni(848<br />
eV) Auger transition lines and the WF change, ΔΦ,<br />
for Ba deposition on the oxygen chemisorbed phase<br />
O(2×1)/Ni(110).<br />
0.0<br />
-0.5<br />
-1.0<br />
-1.5<br />
-2.0<br />
-2.5<br />
-3.0<br />
-3.5<br />
ΔΦ (eV)<br />
3. Results and discussion<br />
The first step was to form the oxygen chemisorbed phase<br />
O(2×1)/Ni(110) surface, by exposing the nickel surface to 2 L of<br />
oxygen. This is the initial adsorption stage of oxygen on the<br />
Ni(110) surface [6]. We then started the Ba deposition on the<br />
oxygenated nickel surface. Figure 1 shows the measured Auger<br />
signal from peak to peak height (AP-PH) for the Ba(590 eV) and<br />
Ni(848 eV) Auger transition lines as well as the change of the<br />
WF, ΔΦ, induced by Ba deposition. A linear increase of the Ba<br />
AES signal is clear, with a change of the slope at periodic<br />
deposition time intervals (14 and 28 min respectively). In parallel,<br />
a linear decrease of the Ni AES signal is observed, with changes<br />
of slope at about the same deposition times. This manner of the<br />
adsorbate and substrate AES signal variation is characteristic of<br />
the layer by layer growth mode. The same growth mode has been<br />
documented for the Ba adsorption on the clean Ni(110) surface<br />
[5]. The first break is attributed to the first physical layer of Ba on<br />
the nickel surface at coverage 0.75 ML, while the second one to the second layer at 1.5 ML. However, a careful look at the<br />
substrate signal decrease at the beginning of the Ba adsorption, shows a small deviation from linearity. This delay of the<br />
Ni(848 eV) signal reduction is probably related to the (2×1) phase, where the oxygen atoms reconstruct the Ni(110) surface.<br />
We believe that the Ba deposition on the O(2×1) phase, lifts the reconstruction of the nickel surface due to the Ba-O<br />
interaction. Indeed, LEED observations showed that the (2×1) symmetry was disappearing even at the very low Ba coverage<br />
(0.1 ML). The WF change, ΔΦ, shown also in Fig. 1, is quite similar to that of the Ba growth on the clean nickel surface<br />
[5,7]. This indicates that Ba adatoms at low coverages are strongly positively polarized due to the reaction with the oxygen<br />
and/or the nickel atoms, while at higher coverages (>0.75 ML) Ba approaches the metallic state.<br />
156